Eight Observations That Are Now Explained
1. Volcanic Gases. By volume, CO2 makes up approximately 20% of all volcanic gases; 70% is steam.8 This water and CO2 came from the subterranean water.
2. Carbon Distribution. Could today’s surface waters have always been at the Earth’s surface while the Earth’s limestone slowly precipitated? Not based on the surprising distribution of carbon on Earth. Table 8 shows that much more carbon exists in limestone than in all other sources combined.
Here is the problem. The above chemical equation shows that for every carbon atom precipitated in limestone, a carbon atom is released in CO2. At the Earth’s surface, this gas enters the biosphere. Had all limestone slowly precipitated in surface waters, as much carbon would have been released into the atmosphere and surface waters (as CO2)
| Place |
Amount of Carbon |
|---|---|
| Atmosphere |
720 |
| Animals and Plants (living and dead) |
2,000 |
| Coal and Oil |
4,130 |
| Oceans (inorganic) |
37,400 |
| Sediments (primarily limestone) |
> 60,000,000 |
as was precipitated in limestone (as CaCO3). Earth’s limestone contains more than 60,000,000 × 1015 grams of carbon. That amount of carbon in the atmosphere and seas would have made them fatally toxic hundreds of times over. Life would have ceased. Today, the atmosphere and seas contain only (720 + 37,400) × 1015 grams of carbon.

Figure 153: Carlsbad Caverns, New Mexico. U.S. Forest Service cave expert, geologist Jerry Trout states, “What geologists used to believe was fact, in terms of dating a cave, now is speculation. ... From 1924 to 1988, there was a visitor’s sign above the entrance to Carlsbad Caverns that said Carlsbad was at least 260 million years old. ... In 1988, the sign was changed to read 7 to 10 million years old. Then, for a little while, the sign read that it was 2 million years old. Now the sign is gone.” Trout also says that geologists don’t know how long cave development takes, and through photo-monitoring, he has watched a stalactite grow several inches in a matter of days.11 [Also see Figure 30 on page 37.]
3. Rapid Stalactite and Stalagmite Formation. Frequently the claim is made that stalactites and stalagmites required millions of years to form. More and more people recognize that this conclusion assumes that these limestone formations always grew at today’s extremely slow rates. [See Figure 30 on page 37 and Figure 153.] With so much water draining through freshly deposited limestone after the flood, stalactites and stalagmites grew rapidly.
Acidic groundwater, plentiful during the centuries after the flood, frequently seeped into cracks in limestone rocks, dissolved limestone, and formed underground caverns. As ventilation in caverns improved and plant growth removed CO2 from the atmosphere, CO2 escaped from this groundwater. Large quantities of limestone precipitated, rapidly forming stalactites and stalagmites worldwide.
4. Organic Limestone. Shallow-water organisms, such as corals, shelled creatures, and some types of algae, remove dissolved limestone from seawater to build hard body parts. (The more abundant the dissolved limestone, the faster the growth rates. Thus, coral growth rates were much higher after the flood.) Because some organisms produce limestone, evolutionists conclude that almost all limestone came from organisms, so hundreds of millions of years are needed to explain thick deposits of limestone. Instead, organic limestone is a result of the presence of inorganic limestone, not its cause. Inorganic limestone precipitated rapidly from the subterranean water before and during the flood. Surface waters could not have held the 60,000,000 × 1015 grams of carbon needed to produce today’s limestone without making them hundreds of times too toxic for sea life to exist.
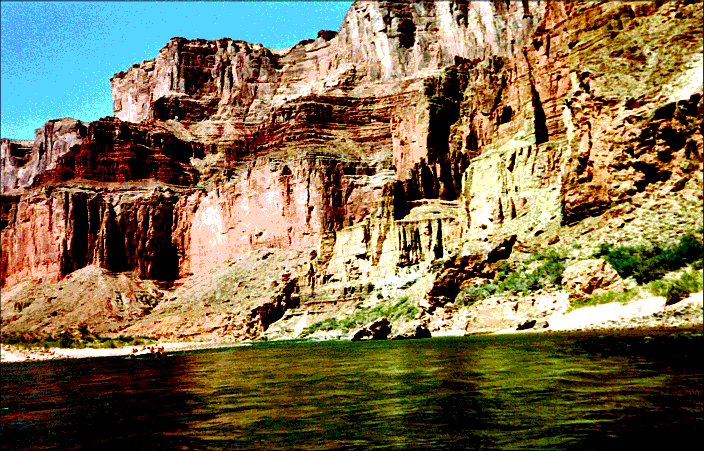
Figure 154: Redwall Limestone Exposed in and around the Grand Canyon. Stained red from iron oxide impurities, the 400-foot-thick Redwall Limestone extends over most of northern Arizona. If it formed in a shallow sea (25–50 feet deep), how did such great thicknesses develop? How could another famous limestone formation, the 6-mile-thick Bahamas Bank, form?
For two other reasons, we can reject the common belief that most limestone has an organic origin. Wave action and predators can fragment shells and other hard parts of marine organisms. However, as fragments become smaller, it is more difficult to break them into smaller pieces. With increasingly smaller pieces, the forces required to break them again become unreasonably large before the pieces reach the size of typical limestone grains.
Finally, organic limestone is structurally different from and more intricate than inorganic limestone. Organic limestone crystals are more uniformly sized, oriented, and packaged—characteristics now detectable with high magnification.10 Earth’s vast limestone layers are overwhelmingly inorganic.
In summary, while much limestone precipitated before and during the flood, seawater still contains dissolved inorganic limestone. Algae, corals, and shelled creatures take in these dissolved chemicals and produce intricate organic limestone.
5. Thick Limestone Banks and Chalk. Scattered off the east coast of the United States are thick limestone deposits. Most dramatic is the Bahamas Bank, an area 250 by 800 miles, where “seismic evidence suggests that carbonate strata may extend down as far as 10 kilometers [6 miles].” 12
If limestone formed organically in shallow seas (the prevailing view), why would the seafloor slowly subside almost 6 miles to allow these accumulations? Subsidence rates would have to be just right during the millions of years needed for organisms to grow and accumulate to such depths. Besides, the seafloor cannot subside unless the rock below it gets out of the way. That rock would have nowhere to go.
Apparently, the flood waters escaping from under the northeastern edge of the Americas hydroplate dumped limestone at the Bahamas Bank.13 Similarly, waters escaping from under the northwestern edge of the European-Asian-African hydroplate dumped limestone in and around what is now the English Channel. Later, in warm surface waters, rich in dissolved limestone, vast algae blooms—perhaps daily—produced the soft, fine-grained type of limestone known as chalk. As long as nutrients and sunlight are plentiful (as was the case following the flood) algae blooms will expand exponentially. The algae die quickly and sink to the bottom of the sea. Most famous are the exposed layers in England’s White Cliffs of Dover and France’s Normandy coast. [See Figure 151 on page 258.]
Some deep-sea sediments include the components of chalk: silicate and calcareous (limestone) structures secreted by tiny organisms, such as foraminifera and coccoliths (a type of algae). Today, when they die, their hard body parts settle to the ocean floor too slowly to (1) bury and fossilize larger animals or (2) achieve the purity seen in famous chalk deposits. Because thick and very pure chalk deposits worldwide preserve many large fossils, including soft-body animals, deposition had to be rapid. Secondly, the microscopic organisms that form chalk must have abundant sources of dissolved limestone and silica—exactly what algae blooms require and the warm waters from the subterranean chambers provided. Powerful wave action, driven by the fluttering crust (explained on page 197) and mountain building events, could have easily scoured, transported, and dumped these low-density sediments into thick, pure, fossil-bearing, chalk deposits.
6. The Dolomite Problem. If a microscopic limestone crystal grows in a magnesium-rich solution, magnesium ions will, under certain conditions, occupy or replace exactly half the calcium ion locations in limestone, forming a common mineral called dolomite.
Geologists frequently refer to “the dolomite problem.” Why is it a problem? Organisms rarely secrete dolomite, certainly not in the quantities needed to account for thick dolomite deposits. If organisms deposited almost all limestone over millions of years, how did dolomite form?
Dolomite is frequently found in contact with limestone and is strangely distributed on Earth. It has hardly ever formed in recent times.14 Therefore, magnesium-rich solutions must have been much more abundant when older rocks were deposited. [See Table 9.]
Some geologists reject precipitation of dolomite because of “the great thicknesses of dolomite rock that are found in the geologic record.”15 Others say that a lot of magnesium-rich water trickled through limestone, but that raises even more problems. How did it trickle so uniformly through such great depths? Why would this “trickling” happen so often near limestone—and primarily in the ancient past? What was the source of the magnesium?
Basalt contains large amounts of magnesium, so the supercritical water dissolved minerals containing magnesium. Therefore, the presence of dolomite near limestone and the even distribution of magnesium in what would otherwise be limestone is easily understood.
| Observations |
Hydroplate Explanations |
|---|---|
| “Dolomites are associated almost exclusively with two other rock types: limestone and evaporites [such as salt].” |
Similar conditions were involved in depositing large amounts of dolomite, salt, and limestone. |
| “Dolomites occur in approximately the same tectonic and physiographic settings as limestones: on the shallow shelves of low-lying continents, most commonly far from the nearest convergent plate margin [ocean trenches].” |
Dolomite and limestone are often found near the edge of a hydroplate. They would rarely be found near ocean trenches (so-called “convergent plate margins”). |
| “[Dolomite] is rare in modern carbonate environments [but is abundant in lower layers].” |
Little dolomite forms today, because the magnesium was released in the subterranean chamber where it was quickly consumed by limestone to form dolomite. |
| “Fossils are noticeably less common in dolomites [than in limestone].” |
Fossils found in limestone are usually organisms that thrive in limy waters: corals, foraminifers, bryozoans, and crinoids. They evidently were buried by postflood deposition of limestone. |
| “The contacts [of dolomites] with limestone above and below are usually sharp.” |
Liquefaction produced sharp contacts. |
7. Worldwide Cement. Evolutionists believe that most limestone was produced organically in shallow seas, because corals and shelled creatures live in shallow seas, which are generally warmer and have higher evaporation rates. With greater evaporation, the remaining solution is more likely to reach concentrations at which organisms can produce shells and other forms of limestone.
Organic limestone is primarily produced within 30 degrees of the equator. However, limestone layers and cement are not concentrated near the equator. Rocks, cemented with limestone, are found at all latitudes. Obviously, whatever produced inorganic limestone was global in scope.
8. Limestone and Silica Cement. As dissolved CO2 slowly escaped the flood waters, limestone and quartz precipitated into the tiniest cracks it could find. In this way, cementing occurred. (This solves “the quartz problem.” 4)
After limestone, silica (SiO2) is the second most common cementing agent in rocks. Derived from quartz, silica dissolves only 6 parts per million in pure water at 77°F (25°C). As temperatures rise, more silica goes into solution. At 300°F (150°C), silica concentrations reach 140 parts per million. If a silica-rich solution occupied the pore space between sand grains, silica would precipitate on their solid surfaces as the water cooled, cementing loose grains into rocks.
Only under high pressure can water reach such high temperatures. The hydroplate theory shows how both high temperature and pressure conditions existed in the subterranean chamber. [See page 126.] Also, frictional sliding of deep rock surfaces and plastic deformations generated enormous heat, which melted rock, forming magma. These hot surfaces heated deep, high-pressure water containing abundant quartz grains.
Sediments fell through silica-rich water. Therefore, the cementing solution was automatically in place between deposited sedimentary particles. It is difficult to imagine another scenario in which so much superheated liquid water could dissolve silica, distribute silica-rich solutions worldwide, and then, before they cooled, force them down into sediments where cementing could occur.
Silica also plays a role in the petrification of wood. As the flood waters drained, continental basins became lakes. Trees floating in warm postflood lakes often became saturated with silica-rich solutions. Petrification occurred as the water cooled and silica precipitated on cellulose surfaces. Petrification has been duplicated in the laboratory when silica concentrations reach 140 parts per million.17 Arizona’s famous petrified forest lies in the center of what was Hopi Lake, while the petrified logs in Utah’s Escalante Petrified Forest and along the Green River both lie in what was Grand Lake. The sudden emptying of both lakes eroded the Grand Canyon. [For many more details about these lakes, petrified wood, and the formation of the Grand Canyon, see pages 215–247.]