Below is the online edition of In the Beginning: Compelling Evidence for Creation and the Flood,
by Dr. Walt Brown. Copyright © Center for Scientific Creation. All rights reserved.
Click here to order the hardbound 8th edition (2008) and other materials.
The Origin of Earth’s Radioactivity
SUMMARY: As the flood began, stresses in the massive fluttering crust generated huge voltages via the piezoelectric effect.4 For weeks, powerful electrical surges within earth’s crust—much like bolts of lightning—produced equally powerful magnetic forces that squeezed (according to Faraday’s Law) atomic nuclei together into highly unstable, superheavy elements. Those superheavy elements quickly fissioned and decayed into subatomic particles and various isotopes, some of which were radioactive.
Each step in this process is demonstrable on a small scale. Calculations and other evidence show that these events happened on a global scale.5 To quickly understand what happened, see “Earthquakes and Electricity” on page 393 and Figures 202and 207–209.
Evolutionists say earth’s radioactive material evolved in stars and their exploded debris. Billions of years later, the earth formed from that debris. Few of the theorized steps can be demonstrated experimentally. Observations on earth and in space support the hydroplate explanation and refute the evolution explanation for earth’s radioactivity.
To contrast and evaluate two radically different explanations for the origin of earth’s radioactivity, we will first explain some terms. With that background, new and surprising experimental evidence will become clear. Next, the two competing theories will be summarized: the hydroplate theory and the chemical evolution theory. Readers can then judge for themselves which theory better explains the evidence. First, we need to understand a few terms concerning the atom.
The Atom. Descriptions and models of the atom differ. What is certain is that no model proposed so far is completely correct.6 Fortunately, we need not consider these uncertainties here. Let us think of an atom as simply a nucleus surrounded by one or more shells—like layers of an onion. Each shell can hold a certain number of negative charges called electrons. (The innermost shell, for example, can hold two electrons.) The tightly packed, vibrating nucleus contains protons, each with a positive charge, and neutrons, with no charge. (Protons and neutrons are called nucleons.)
An atom is small. Two trillion (2,000,000,000,000, or 2 × 1012 ) carbon atoms would fit inside the period at the end of this sentence. A nucleus is even smaller. If an atom were the size of a football field, its nucleus—which contains about 99.98% of an atom’s mass—would be the size of a tiny seed ! Electrons are smaller yet. An electron is to a speck of dust as a speck of dust is to the earth!
Atoms of the same chemical element have the same number of protons. For example, a hydrogen atom has one proton; helium, two; lithium, three; carbon, six; oxygen, eight; iron, 26; gold, 79; and uranium, 92. Today, earth has 94 naturally occurring chemical elements.7
A carbon-12 atom, by definition, has exactly 12.000000 atomic mass units (AMU). If we could break a carbon-12 atom apart and “weigh” each of its six protons, six neutrons, and six electrons, the sum of their masses would be 12.098940 AMU—which is 0.098940 AMU heavier than the carbon-12 atom itself. To see why an atom weighs less than the sum of its parts, we must understand binding energy.
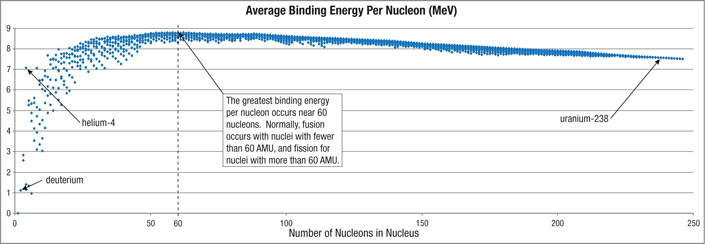
Figure 201: Binding Energy. When separate nucleons (protons and neutrons) are brought together to form a nucleus, a tiny percentage of their mass is instantly converted to a large amount of energy. That energy (usually measured in units of millions of electron volts, or MeV) is called binding energy, because an extremely strong force inside the nucleus tightly binds the nucleons together—snaps them powerfully together—producing a burst of heat.
For example, a deuterium (hydrogen-2) nucleus contains a proton and a neutron. Its nucleus has a total binding energy of about 2.2 MeV, so the average binding energy per nucleon is about 1.1 MeV. If two deuterium nuclei merge to become helium, 2.2 MeV + 2.2 MeV of binding energy are replaced by helium-4’s average binding energy of 7.1 MeV per nucleon, or a total of 4 x 7.1 MeV. The gain in binding energy becomes emitted heat. This merging of light nuclei is called fusion. The Sun derives most of its heat by the fusion of deuterium into helium.8 The peak of the binding energy curve (above) is around 60 AMU (near iron), so fusion normally9 merges into nuclei lighter than 60 AMU. The fusion of elements heavier than 60 AMU absorb energy.
Fission is the splitting of heavy nuclei. For example, when uranium fissions, the sum of the binding energies of the fragments is greater than the binding energy of the uranium nucleus, so energy is released. Fission (as well as fusion) can be sustained only if energy is released to drive more fission (or fusion).
Binding Energy. When a nucleus forms, a small amount of mass is converted to binding energy, the energy emitted by the nucleus when protons and neutrons bind together. It is also the energy required to break (unbind) a nucleus into separate protons and neutrons.
The closer the mass of a nucleus is to the mass of an iron or nickel nucleus (60 AMU), the more binding energy that nucleus has per nucleon. Let’s say that a very heavy nucleus, such as a uranium nucleus weighing 235.0 AMU, splits (fissions) into two nuclei weighing 100.0 AMU and 133.9 AMU and a neutron (1.0 AMU). The 0.1 AMU of lost mass is converted to energy, according to Einstein’s famous equation, E = m c2, where c is the speed of light (186,000 miles per second) and E is the energy released when a mass m is converted to energy. The energy is great, because c2 is huge. (For example, when the atomic bomb was dropped on Hiroshima, only about 700 milligrams of mass—about one-third the mass of a U.S. dime—was converted to energy.) Nuclear energy is usually released as kinetic energy. The high velocity fragments generate heat as they slow down during multiple collisions.
Stated another way, a very heavy nucleus sometimes splits, a process called fission. (Fission may occur when a heavy nucleus is hit by a neutron, or even a high-energy photon (particle of light). When fission happens spontaneously—without being hit—it is a type of decay. When fission occurs, mass is lost and energy is released. Likewise, when light nuclei merge (a process called fusion), mass is lost and energy is released. In an atom bomb, uranium or plutonium nuclei split (fission). In a hydrogen bomb, hydrogen nuclei merge (fuse) to become helium.
Fission inside nuclear reactors produces many free neutrons. Water is an excellent substance for absorbing the energy of fast neutrons and thereby producing heat, because water is cheap and contains so much hydrogen. (A hydrogen atom has about the same mass as a neutron, so hydrogen quickly absorbs a fast neutron’s kinetic energy.) The heat can then boil water to produce steam that spins a turbine and generates electricity.
Isotopes. Chemical elements with the same number of protons but a different number of neutrons are called isotopes. Every chemical element has several isotopes, although most are seen only briefly in experiments. Carbon-12, carbon-13, and carbon-14 are different isotopes of carbon. All are carbon, because they have 6 protons, but respectively, they have 6, 7, and 8 neutrons—or 12, 13, and 14 nucleons. The number of protons determines the chemical element; the number of neutrons determines the isotope of the element.
Radioactivity. Most isotopes are radioactive; that is, their vibrating, unstable nuclei sometimes change spontaneously (decay), usually by emitting fast, very tiny particles—even photons (particles of light) called gamma rays. Each decay, except gamma emission, converts the nucleus into a new isotope, called the daughter. One type of radioactive decay occurs when a nucleus expels an alpha particle—a tight bundle of two protons and two neutrons, identical to the nucleus of a helium atom. In another type of decay, beta decay, a neutron suddenly emits an electron and becomes a proton. Electron capture, a type of decay, is beta decay in reverse; that is, an atom’s electron enters the nucleus, combines with a proton, and converts it into a neutron. Few scientists realize that on rare occasions heavy nuclei will decay by emitting a carbon-14 nucleus (14C).13 This calls into question the basic assumptions of the radiocarbon dating technique, especially when one understands the origin of earth’s radioactivity. [See "How Accurate Is Radiocarbon Dating?" on pages 523–527.]
Radioisotopes. Radioactive isotopes are called radioisotopes. Only about 65 naturally occurring radioisotopes are known. However, high-energy processes (such as those occurring in atomic explosions, atomic accelerators, and nuclear reactors) have produced about 3,000 different radioisotopes, including a few previously unknown chemical elements.
Decay Rates. Each radioisotope has a half-life—the time it would take for half of a large sample of that isotope to decay at today’s rate. Half-lives range from less than a billionth of a second to many millions of trillions of years.14 Most attempts to change decay rates have failed. For example, changing temperatures between -427°F and +4,500°F has produced no measurable change in decay rates. Nor have accelerations of up to 970,000 g, magnetic fields up to 45,000 gauss, or changing elevations or chemical concentrations.
However, it was learned as far back as 1971 that high pressure could increase decay rates very slightly for at least 14 isotopes.15 Under great pressure, electrons (especially from the innermost shell) are squeezed closer to the nucleus, making electron capture more likely. Also, electron capture rates for a few radioisotopes change in different chemical compounds.16
Beta decay rates can increase dramatically when atoms are stripped of all their electrons. In 1999, Germany’s Dr. Fritz Bosch showed that, for the rhenium atom, this “decreases its half-life more than a billionfold—from 42-billion years to 33 years.”17 The more electrons removed, the more rapidly neutrons expel electrons (beta decay) and become protons. This effect was previously unknown, because only electrically neutral atoms had been used in measuring half-lives.18
Decay rates for silicon-32 (32Si), chlorine-36 (36Cl), manganese-54 (54Mn), and radium-226 (226Ra) depend slightly on earth’s distance from the Sun.19 They decay, respectively, by beta, alpha, and electron capture. Other radioisotopes seem to be similarly affected. This may be an electrical effect or a consequence of neutrinos20 flowing from the Sun.
Patents have been awarded to major corporations for electrical devices that claim to accelerate alpha, beta, and gamma decay and thereby decontaminate hazardous nuclear wastes. However, they have not been shown to work on a large scale. An interesting patent awarded to William A. Barker is described as follows:21
Radioactive material is placed in or on a Van de Graaff generator where an electric potential of 50,000 – 500,000 volts is applied for at least 30 minutes. This large negative voltage is thought to lower each nucleus’ energy barrier. Thus alpha, beta, and gamma particles rapidly escape radioactive nuclei.
While these electrical devices may accelerate decay rates, a complete theoretical understanding of them does not yet exist, they are expensive, and they act only on small samples. However, the common belief that decay rates are constant in all conditions should now be discarded.
We can think of a large sample of a radioisotope as a slowly-leaking balloon with a meter that measures the balloon’s total leakage since it was filled. Different radioisotopes have different leakage rates, or half-lives. (Stable isotopes do not leak; they are not radioactive.)
Some people may think that a balloon’s age can be determined by dividing the balloon’s total leakage by its leakage rate today. Here, we will address more basic issues: What “pumped up” all radioisotopes in the first place, and when did it happen? Did the pumping process rapidly produce considerable initial leakage—billions of years’ worth, based on today’s slow leakage rates?
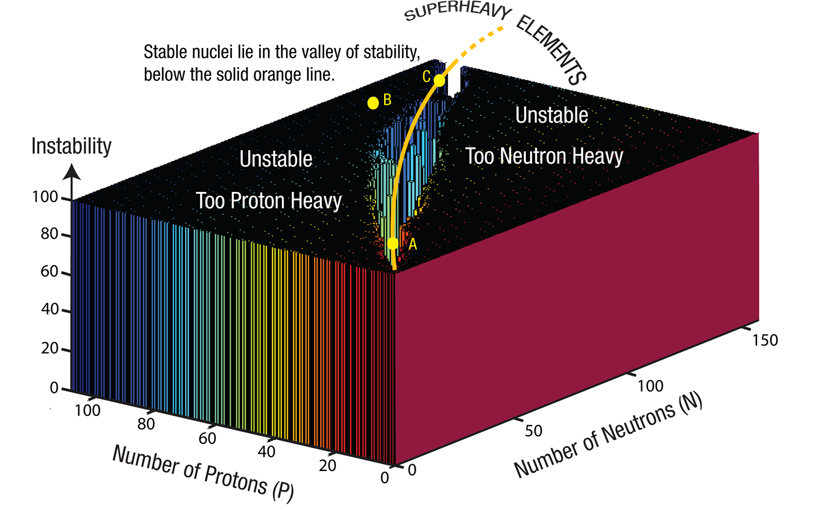
Figure 202: Valley of Stability.
Each of the more than 3,100 known isotopes is defined by two numbers: the number of protons (P) and the number of neutrons (N). Think of each isotope as occupying a point on a horizontal P–N coordinate system. There, each isotope’s stability can be represented by a thin, vertical bar: tall bars for isotopes that decay rapidly, shorter bars for isotopes with longer half-lives, and no vertical bars for stable isotopes.10 Almost 300 stable isotopes are represented far below the curved orange line, in what is called the valley of stability. It lies near the diagonal between the P axis and the N axis.
Almost all isotopes represented by the high, flat “plateau” are hypothetical and have never been seen, but if they ever formed, they would decay instantly. Most of the thousand or so isotopes briefly observed in experiments lie just below the edge of the “cliff” looking down into the valley. Those on the steep slope have half-lives of seconds to billions of years. Stable isotopes are down on the valley floor.
Notice how the valley curves toward the right.11 Light, stable nuclei have about the same number of protons as neutrons (such as carbon-12 with six protons and six neutrons); heavy nuclei that are stable have many more neutrons than protons. A key point to remember: if we could squeeze several light, stable nuclei together to make one heavy nucleus, it would lie high on the proton-heavy side of the valley, be radioactive, and would soon decay.
For example, if some powerful compression or the Z-pinch (described in Figure 200 on page 386) suddenly merged (fused) six stable nuclei near point A, the resulting heavy nucleus would briefly lie at point B, where it would quickly decay or fission.12 Merged nuclei that were even heavier—superheavy nuclei—would momentarily lie far beyond point B, but would instantly fission—fragment into many of our common chemical elements. If the valley of stability were straight and did not curve, stable nuclei that fused together would form a stable, heavy nucleus (i.e., would still lie on the valley floor). Nuclei near C that fission will usually produce neutron-heavy products. As you will see, because the valley curves, we have radioactivity—another key point to remember. (Soon, you will learn about the “strong force” which produces binding energy and causes the valley to curve.)
If all earth’s nuclei were initially nonradioactive, they would all have been at the bottom of the curved valley of stability. If, for weeks, chaotic discharges of electrons, driven by billions of volts of electricity, pulsed through the earth’s crust, radioactive isotopes and their decay and fission products would quickly form. (How this happened will be explained later.) We can think of these new isotopes as being scattered high on the sides of the valley of stability.
It would be as if a powerful explosion, or some sudden release of energy, blasted rocks up onto the steep sides of a long valley. Most rocks would quickly roll back down and dislodge somewhat unstable rocks that were only part way up the slope. Today, rocks rarely roll down the sides of the valley. Wouldn’t it be foolish to assume that the rubble at the bottom of this valley must have been accumulating for billions of years, merely because it would take billions of years for all that rubble to collect at the very slow rate rocks roll down today?
Later in this chapter, you will see the well-established physical processes that —in less than one hour—greatly accelerated radioactive decay during the flood.
Neutron Activation Analysis. This routine, nondestructive technique can be used to identify chemical elements in an unknown material. Neutrons, usually from a nuclear reactor, bombard the material. Some nuclei that absorb neutrons become radioactive—are driven up the neutron-heavy side of the valley of stability. [See Figure 202 on page 390.] The decay characteristics of those “pumped up” nuclei then help identify the atoms present.
Neutron Stars. When a very massive star begins to run out of hydrogen and other nuclear fuels, it can collapse so suddenly that almost all its electrons are driven into nuclei. This produces a “sea of neutrons” and releases the immense energy of a supernova. What remains near the center of the gigantic explosion is a dense star, about 10 miles in diameter, composed of neutrons—a neutron star.
The Strong Force. Like charges repel each other, so what keeps a nucleus containing many positively charged protons from flying apart? A poorly understood force inside the nucleus acts over a very short distance to pull protons (and, it turns out, neutrons, as well) together. Nuclear physicists call this the strong force. Binding energy, described on page 388, is the result of work done by the strong force.
Two nuclei, pushed toward each other, initially experience an increasing repelling force, called the Coulomb force, because both nuclei have positive charges. However, if a voltage is accelerating many nuclei in one direction and electrons are flowing between them in the opposite direction, that repelling force is largely neutralized. Furthermore, both positive and negative flows will produce a reinforcing Z-pinch. [See Figure 200 on page 386.] If the voltage driving both flows is large enough, the Z-pinch brings the two nuclei close enough together so that the strong force merges them into one large nucleus.22
If the Z-pinch acts over a broad plasma flow, many nuclei could merge into superheavy nuclei—nuclei much heavier than any chemical element found naturally. Most merged nuclei would be unstable (radioactive) and would rapidly decay, because they would lie high on the proton-heavy side of the valley of stability. [See Figure 202 on page 390.]
While the strong force holds nuclei together and overcomes the repelling Coulomb force, four particular nuclei are barely held together: lithium-6 (6Li), beryllium-9 (9Be), boron-10 (10B), and boron-11 (11B). Slight impacts will cause their decay.23 The importance of these fragile isotopes will soon become clear.
Free Neutrons. Neutrons in a nucleus rarely decay, but free neutrons (those outside a nucleus) decay with a half-life of about 14.7 minutes! Why should a neutron surrounded by protons and electrons often have a half-life of millions of years, but, when isolated, have a half-life of minutes? 24 This is similar to what Fritz Bosch discovered: When an intense electric field strips electrons surrounding certain heavy nuclei, those nuclei become so unstable that their decay rate increases, sometimes a billionfold.
Carbon-14. Each year, cosmic radiation striking the upper atmosphere converts about 21 pounds of nitrogen-14 into carbon-14, also called radiocarbon. Carbon-14 has a half-life of 5,730 years. Radiocarbon dating has become much more precise, by using Accelerator Mass Spectrometry (AMS), a technique that counts individual carbon-14 atoms. AMS ages for old carbon-14 specimens are generally about 5,000 years. [See “How Accurate Is Radiocarbon Dating?” on pages 523–527.] AMS sometimes dates the same materials that were already dated by older, less-precise radiometric dating techniques. In those cases, AMS ages are usually 10–1000 times younger.25
Argon-40. About 1% of earth’s atmosphere (not counting water vapor) is argon, of which 99.6% is argon-40 and only 0.3% is argon-36. Both are stable. Today, argon-40 is produced almost entirely by electron capture in potassium-40. In 1966, Melvin Cook pointed out the great discrepancy in the large amount of argon-40 in our atmosphere, the relatively small amount of potassium-40 in the earth’s crust, and its slow rate of decay (half-life: 1.3-billion years).
The earth would have to be about 1010 years old [10-billion years, twice what evolutionists believe] and the initial 40K [potassium-40] content of the earth about 100 times greater than at present ... to have generated the 40Ar [argon-40] in the atmosphere.26
Since Cook published that statement, estimates of the amount of 40K in the earth have increased. Nevertheless, a glaring contradiction remains. Despite geophysicists’ efforts to juggle the numbers, the small amount of 40K in the earth is not enough to have produced all the 40Ar, the fourth most abundant gas in the atmosphere (after nitrogen, oxygen, and water vapor). If 40Ar was produced by a process other than the slow decay of 40K, as the evidence indicates, then the potassium-argon and argon-argon dating techniques, the most frequently used radiometric dating techniques,27 become useless, if not deceptive.
Likewise, Saturn’s icy moon Enceladus has little 40K but is jetting too much 40Ar into space from its south pole. Enceladus would need a thousand times its current rock content consisting of the most favorable types of meteorites to explain all the argon-40.28 Even with that much 40K, how would the argon rapidly escape from the rock and be concentrated? In the previous chapter, evidence was given showing that Enceladus and other irregular moons in the solar system are captured asteroids, whose material was expelled from earth by the fountains of the great deep. Could all that 40Ar have been produced in the subterranean chamber and expelled as part of the debris? Enceladus also contains too much deuterium—about the same amount as in almost all comets and more than ten times the concentration found in the rest of the solar system.29 This was explained in the comet chapter as one of seventeen major reasons for concluding that the material in comets was launched from earth by the fountains of the great deep.
One final point: Micrometeorites and solar wind add at least seven times more 36Ar than 40Ar to earth’s atmosphere. Therefore, those sources provided little of the earth’s 40Ar,30 because, as stated above, our atmosphere has about 300 times more 40Ar than 36Ar.
Potassium-40 and Carbon-14. Potassium-40 is the most abundant radioactive substance in the human body and in every living thing. (Yes, your body is slightly radioactive!) Fortunately, potassium-40 decays by expelling an electron (beta decay) which is not very penetrating. Nevertheless, when potassium-40 decays it becomes calcium, so if the tiny electron “bullet” didn’t damage you, the sudden change from potassium to calcium could be quite damaging—almost as if a screw in a complex machine suddenly became a nail. While only one ten-thousandth of the potassium in living things is potassium-40, most has already decayed, so living things were at greater risk in the past. How could life have evolved if it had been radioactive?”
That question also applies for the rare radioactive isotopes in the chemical elements that are in DNA, such as carbon-14. DNA is the most complex material known. A 160-pound person experiences 2,500 carbon-14 disintegrations each second, almost 10 of which occur each second in the person’s DNA! [See Endnote 4 on page 529.]
The answer to this question is simple. Life did not evolve, and earth’s radioactivity was not present when life began. Earth’s radioactivity is a consequence of the flood. [See "Mutations" on page 9.]
Zircons. Zircons are tiny, durable crystals about twice the thickness of a human hair. They usually contain small amounts of uranium and thorium, some of which is assumed to have decayed, at today’s very slow rates, to lead. If this is true, zircons are extremely old. For example, hundreds of zircons found in Western Australia would be 4.0–4.4-billion years old. Most evolutionists find this puzzling, because they have claimed that the earth was largely molten prior to 3.9-billion years ago!37 These zircons also contain tiny inclusions of quartz, which suggests that the quartz was transported in and precipitated out of liquid water; if so, the earth was relatively cool and had a granite crust.38 Other zircons, some supposedly as old as 4.42-billion years, contain microdiamonds with abnormally low, but highly variable amounts of 13C. These microdiamonds apparently formed (1) under unusual geological conditions, and (2) under extremely high, and perhaps sudden, pressures before the zircons encased them.39
Helium Retention in Zircons. Uranium and thorium usually decay by emitting alpha particles. Each alpha particle is a helium nucleus that quickly attracts two electrons and becomes a helium atom (4He). The helium gas produced in zircons by uranium and thorium decay should diffuse out relatively quickly, because helium does not combine chemically with other atoms, and it is extremely small—the second smallest of all elements by mass, and the smallest by volume!
Some zircons would be 1.5-billion years old if the lead in them accumulated at today’s rate. But based on the rapid diffusion of helium out of zircons, the lead would have been produced in the last 4,000–8,000 years40—a clear contradiction, suggesting that at least one time in the past, rates were faster.
Helium-3 (3He). Ejected alpha particles, as stated above, quickly become 4He, which constitutes 99.999863% of the earth’s detectable helium. Only nuclear reactions produce 3He, the remaining 0.000137% of earth’s known helium. Today, no nuclear reactions are known to produce 3He inside the earth. Only the hydroplate theory explains how nuclear reactions produced 3He at one time (during the flood) inside the solid earth (in the fluttering crust).41
3He and 4He are stable (not radioactive). Because nuclear reactions that produce 3He are not known to be occurring inside the earth, some evolutionists say that 3He must have been primordial—present before the earth evolved. Therefore, 3He, they say, was trapped in the infalling meteoritic material that formed the earth. But helium does not combine chemically with anything, so how did such a light, volatile gas get inside meteorites? If helium was trapped in falling meteorites, why did it not quickly escape or bubble out when meteorites supposedly crashed into the molten, evolving earth?42 If 3He is being produced inside the earth and the mantle has been circulating and mixing for millions of years, why do different volcanoes expel drastically different amounts of 3He, and why—as explained in Figure 56 on page 127—are black smokers expelling large amounts of 3He?43Indeed, the small amount of 3He should be so thoroughly mixed and diluted in the circulating mantle that it should be undetectable.44
Where Is Earth’s Radioactivity? Three types of measurements each show that earth’s radioactivity is concentrated in the relatively thin continental (granite) crust. In 1906, some scientists recognized that just the heat from the radioactivity in the granite crust should explain all the heat now coming out of the earth. If radioactivity were occurring below the crust, even more heat should be exiting. Because it is not, radioactivity should be concentrated in the top “few tens of kilometers” of the earth—and have begun recently.
The distribution of radioactive material with depth is unknown, but amounts of the order of those observed at the surface must be confined to a relatively thin layer below the Earth’s surface of the order of a few tens of kilometers in thickness, otherwise more heat would be generated than can be accounted for by the observed loss from the surface.45
Later, holes drilled into the ocean floor showed slightly more heat coming up through the ocean floors than through the continents. But basaltic rocks under the ocean floor contain little radioactivity.46 Apparently, radioactive decay is not the primary source of earth’s geothermal heat.
A second type of measurement occurred in Germany’s Deep Drilling Program. The concentration of radioactivity measured down Germany’s deepest hole (5.7 miles) would account for all the heat flowing out at the earth’s surface if that concentration continued down to a depth of only 18.8 miles and if the crust were 4-billion years old.47
However, the rate at which temperatures increased with depth was so great that if the trend continued, the rock at the top of the mantle would be partially melted. Seismic studies have shown that this is not the case.48 Therefore, temperatures do not continue increasing down to the mantle, so the source of the heating is concentrated in the earth’s crust.
A third measurement technique, used in regions of the United States and Australia, shows a strange, but well-verified, correlation: the amount of heat flowing out of the earth at specific locations correlates with the radioactivity in surface rocks at those locations. Wherever radioactivity is high, the heat flow will usually be high; wherever radioactivity is low, the heat flow will usually be low. However, the radioactivity at those hotter locations is far too small to account for that heat.49 What does this correlation mean?
First, consider what it does not necessarily mean. When two sets of measurements correlate (or correspond), people often mistakenly conclude that one of the things measured (such as radioactivity in surface rocks at one location) caused the other thing being measured (surface heat flow at that location). Even experienced researchers sometimes make this mistake. Students of statistics are repeatedly warned of this common mistake in logic, and hundreds of humorous50 and tragic examples are given; nevertheless, the problem abounds in all research fields.
This correlation could be explained if most of the heat flowing up through the earth’s surface was generated, not by the radioactivity itself, but by the events that produced that radioactivity. If more heat is coming out of the ground at one place, then more radioactivity was also produced there. Therefore, radioactivity in surface rocks would correlate with surface heat flow.
The Oklo Natural “Reactor.” Building a nuclear reactor requires the careful design of many interrelated components. Reactors generate heat by the controlled fission of certain isotopes, such as uranium-235 (235U). For some unknown reason, 0.72% of almost every uranium ore deposit in the world is 235U. (About 99.27% is the more stable 238U, and 0.01% is 234U.) For a 235U reactor to operate, the 235U must usually be concentrated to at least 3–5%. This enrichment is both expensive and technically difficult.
Controlling the reactor is a second requirement. When a neutron splits a 235U nucleus, heat and typically two or three other neutrons are released. If the 235U is sufficiently concentrated and, on average, exactly one of those two or three neutrons fissions another 235U nucleus, the reaction continues and is said to be critical—or self-sustaining. If this delicate situation can be maintained, considerable heat (from binding energy) is steadily released, usually for years.
In 1972, French engineers were processing uranium ore from an open-pit mine near the Oklo River in the Gabon Republic on Africa’s west equatorial coast. There, they discovered depleted (partially consumed) 235U in isolated zones.51 (In one zone, only 0.29% of the uranium was 235U, instead of the expected 0.72%.) Many fission products from 235U were mixed with the depleted 235U but found nowhere else.
Nuclear engineers, aware of just how difficult it is to design and build a nuclear reactor, are amazed by what they believe was a naturally occurring reactor. But notice, we do not know that a self-sustaining, critical reactor operated at Oklo. All we know is that considerable 235U has fissioned.
How could this have happened? Suppose, as is true for every other known uranium mine, Oklo’s uranium layer was never critical. That is, for every 100 neutrons produced by 235U fission, 99 or fewer other neutrons were produced in the next fission cycle, an instant later. The nuclear reaction would quickly die down; i.e., it would not be self-sustaining. However, suppose (as will soon be explained) many free neutrons frequently appeared somewhere in the uranium ore layer. Although the nuclear reaction would not be self-sustaining, the process would multiply the number of neutrons available to fission 235U.52 This would better match what is found at Oklo for four reasons.
First, in several “reactor” zones the ore layer was too thin to become critical. Too many neutrons would have escaped or been absorbed by all the nonfissioning material (called poisons) mixed in with the uranium.53
Second, one zone lies 30 kilometers from the other zones. Whatever strange events at Oklo depleted 235U in 16 largely separated zones was probably common to that region of Africa and not to some specific topography. Uranium deposits are found in many diverse regions worldwide, and yet, only in the Oklo region has this mystery been observed.
Third, depleted 235U was found where it should not be—near the borders of the ore deposit, where neutrons would tend to escape, instead of fission 235U. Had Oklo been a reactor, depleted 235U should be concentrated near the center of the ore body.54
Fourth, at Oklo, the ratio of 235U to 238U in uranium ore, which should be about 0.72 to 99.27 (or 1 to 138), surprisingly varies a thousandfold over distances as small as 0.0004 inch (0.01 mm)!55 A. A. Harms has explained that this wide variation
represents strong evidence that, rather than being a [thermally] static event, Oklo represented a highly dynamic—indeed, possibly “chaotic” and “pulsing” —phenomenon.58
Harms also explained why rapid spikes in temperature and nuclear power would produce a wide range in the ratios of 235U to 238U over very short distances. The question which will soon be answered is, what could have caused those spikes?
Radiohalos. An alpha particle shot from a radioisotope inside a rock acts like a tiny bullet crashing through the surrounding crystalline structure. The “bullet” travels for a specific distance (usually a few ten-thousandths of an inch) depending on the particular radioisotope and the resistance of the crystals it penetrates. If a billion copies of the same radioisotope are clustered near a microscopic point, their randomly directed “bullets” will begin to form a tiny sphere of discoloration and radiation damage called a radiohalo.59
For example, 238U, after a series of eight alpha decays (and six much less-damaging beta decays), will become lead-206 (206Pb). Therefore, eight concentric spheres, each with a slightly different color, will surround what was a point concentration of a billion 238U atoms. Under a microscope, those radiohalos look like the rings of a tiny onion. [See Figure 205.] A thin slice through the center of this “onion” resembles a bull’s-eye target at an archery range. Each ring’s relative size identifies the radio isotope that produced it.
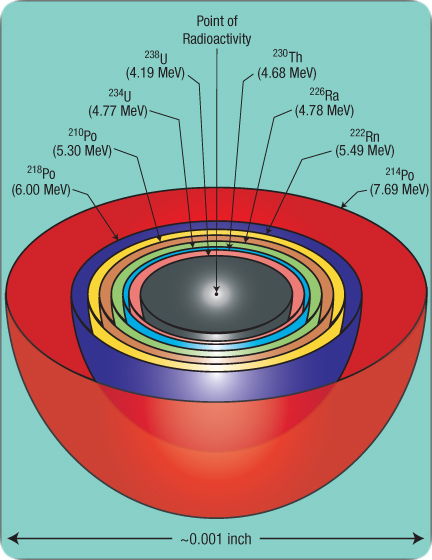
Figure 205: Radiohalos from the 238U Decay Series. Suppose many 238U atoms were concentrated at the point of radioactivity shown here. Each 238U atom eventually ejects one alpha particle in a random direction, but at the specific velocity corresponding to 4.19 million electron volts (MeV) of energy—the binding energy released when 238U decays. That energy determines the distance traveled, so each alpha particle from 238U ends up at the gray spherical shell shown above. (Alpha particles from daughter isotopes will travel to different shells.) Each sharply defined halo requires the ejection of about a billion alpha particles from the common center of all halos, because each alpha particle leaves such a thin path of destruction.
A 238U atom becomes 234U after the alpha decay and two less-damaging beta decays. Later, that 234U atom expels an alpha particle with 4.77 MeV of kinetic energy. As a billion 234U atoms decay, a sharp 234U halo forms. Eventually, a billion lead-206 (206Pb) atoms will occupy the halo center, and each halo’s radius will identify which of the eight radioisotopes produced it.
While we might expect all eight halos to be nested (have a common center) as shown above, G. H. Henderson made a surprising discovery64 in 1939: halos formed by the decay of three polonium isotopes (218Po, 214Po, and 210Po) were often isolated, not nested. Since then, the mystery has deepened, and possible explanations have generated heated controversy.
Thorium-232 (232Th) and 235U also occur naturally in rocks, and each begins a different decay series that produces different polonium isotopes. However, only the 238U series produces isolated polonium halos. Why are isolated polonium halos in the 238U decay series but not in other decay series? If the earth is 4.5-billion years old and 235U was produced and scattered by some supernova billions of years earlier, 235U’s half-life of 700-million years is relatively short. Why then is 235U still around, how did it get here, what concentrated it, and where is all the lead that the 235U decay series should have produced?
Isolated Polonium Halos. We can think of the eight alpha decays from 238U to 206Pb as producing the nine rungs on a generational ladder. Each alpha decay leads to the radioisotope on the ladder’s next lower rung. The last three alpha decays60 are of the chemical element polonium (Po): 218Po, 214Po, and 210Po. Their half-lives are extremely short: 3.1 minutes, 0.000164 second, and 138 days, respectively.
Surprisingly, polonium radiohalos are often found without their parents—or any other prior generation! How could that be? Polonium is always a decay product. It must have had parents! Notice that 222Rn is on the rung immediately above the three polonium isotopes, but the 222Rn halo is missing. Because 222Rn decays with a half-life of only 3.8 days, its halo should be found with the polonium halos. Or should it?
Dr. Robert V. Gentry, the world’s leading researcher on radiohalos, has proposed the following explanation for this mystery.61 He correctly notes that halos cannot form in a liquid, so they could not have formed while the rock was solidifying from a molten state. Furthermore, any polonium in the molten rock would have decayed long before the liquid could cool enough to solidify. Therefore, we can all see that those rocks did not cool and solidify over eons, as commonly taught! However, Gentry believes, incorrectly, that on Day 1 of the creation, a billion or so polonium atoms were concentrated at each of many points in rock; then, within days, the polonium decayed and formed isolated (parentless) halos.
Gentry’s explanation has five problems. First, it doesn’t explain why a billion or so polonium atoms would be concentrated at each of trillions of points that would later become the centers of parentless polonium halos. Second, to form a distinct 218Po halo, those 218Po atoms, must undergo heat-releasing alpha decays, half of which would occur within 3.1 minutes. The great heat generated in such a tiny volume in just 3.1 minutes would have easily melted and erased that entire halo.62 Not only did melting not occur, had the temperature of the halo ever exceeded 300°F (150°C) the alpha tracks would have been erased (annealed).63 Obviously, an efficient heat removal mechanism, which will soon be explained, must have acted.
Third, polonium has 33 known radioisotopes, but only three (218Po, 214Po, and 210Po) account for almost all the isolated polonium halos. Those three are produced only by the 238U decay series, and 238U deposits are often found near isolated polonium halos. Why would only those three isotopes be created instantly on Day 1? This seems unlikely. Instead, something produced by only the 238U decay series accounts for the isolated polonium halos. As you will soon see, that “something” turns out to be 222Rn.
Fourth, Henderson and Sparks, while doing their pioneering work on isolated polonium halos in 1939, made an important discovery: they found that the centers of those halos, at least those in the biotite “books” they examined, were usually concentrated in certain “sheets” inside the biotite.65 (Biotite, like other micas, consists of thin “sheets” that children enjoy peeling off as if the layers were sheets in a book.)
In most cases it appears that they [the centers of the isolated halos] are concentrated in planes parallel to the plane of cleavage. When a book of biotite is split into thin leaves, most of the latter will be blank until a certain depth is reached, when signs of halos become manifest. A number of halos will then be found in a central section in a single leaf, while the leaves on either side of it show off-centre sections of the same halos. The same mode of occurrence is often found at intervals within the book.66
This implies that polonium atoms or their 222Rn parent flowed along what is now the central sheet and lodged in the channel wall as that mineral sheet grew. In other words, the polonium was not created on Day 1 inside solid rock.
Fifth, isolated polonium halos are often found near uranium mines, where magma containing uranium was injected up through fossil bearing strata. Therefore the intrusions and polonium halos obviously came after the flood, which itself was long after creation. The magma slowly cooled and solidified, while the uranium began releasing 222Rn that was quickly dissolved and transported upward in flowing water. The polonium daughters of 222Rn, produced the parentless polonium halos.
On 23 October 1987, after giving a lecture at Waterloo University near Toronto, Ontario, I was approached by amateur geologist J. Richard Wakefield, who offered to show me a similar intrusion. The site was near a uranium mine, about 150 miles to the northeast near Bancroft, Ontario, where Bob Gentry had obtained some samples of isolated polonium halos. I accepted and called my friend Bob Gentry to invite him to join us. Several days later, he flew in from Tennessee and, along with an impartial geologist who specialized in that region of Ontario, we went to the mine. Although we could not gain access into the mine, we all agreed that the intrusion cut up through the sedimentary layers.67
Gentry concluded (while we were there and in later writings68) that the sedimentary layers with solid intrusions must have been created supernaturally with 218Po, 214Po, and 210Po already present (but no other polonium isotopes present). Then the 218Po, 214Po, and 210Po decayed minutes or days later. Unfortunately, I had to disagree with my friend; the heat generated would have melted the entire halo.62 Besides, I am convinced that those sedimentary layers were laid down during the flood, so the intrusions came after the flood and long after the creation, when Gentry claims they formed. [See “Liquefaction: The Origin of Strata and Layered Fossils” on pages 197–214.] Since 1987, isolated polonium halos have been reported in other flood deposits.69
Dr. Lorence G. Collins has a different explanation for the polonium mystery. He first made several perceptive observations. The most important was that strange wormlike patterns were in “all of the granites in which Gentry found polonium halos.”70 Those microscopic patterns, each about 1 millimeter long, resembled almost parallel “underground ant tunnels” and were typically filled with two minerals common in granite: quartz and plagioclase [PLA-jee-uh-clase] feldspars, specifically sodium feldspars.71 The granite had not melted, nor had magma been present. The rock that contains these wormlike patterns is called myrmekite [MUR-muh-kite]. Myrmekites have intrigued geologists and mineralogists since 1875. Collins admits that he does not know why myrmekite is associated with isolated polonium halos in granites.72 You soon will.
Collins notes that those halos all seem to be near uranium deposits and tend to be in two minerals (biotite and fluorite) in granitic pegmatites [PEG-muh-tites] and in biotite in granite when myrmekites are present.73 (Pegmatites will soon be described. Biotite, fluorite, and pegmatites form out of hot water solutions in cracks in rocks.) Collins also knows that radon (Rn) inside the earth’s crust is a gas; under such high pressures, it readily dissolves in hot water. Because radon is inert, it can move freely through solid cracks without combining chemically with minerals lining the walls of those cracks.
Collins correctly concludes that “voluminous” amounts of hot, 222Rn-rich water must have surged up through sheared and fractured rocks.74 When 222Rn decayed, 218Po formed. Collins insights end there, but they raise six questions.
a. What was the source of all that hot, flowing water, and how could it flow so rapidly up through rock?75
b. Why was the water 222Rn rich? 222Rn has a half-life of only 3.8 days!
c. Because halos are found in different geologic periods, did all this remarkable activity occur repeatedly, but at intervals of millions of years? If so, how?
d. What concentrated a billion or so 218Po atoms at each microscopic speck that became the center of an isolated polonium halo? Why wasn’t the 218Po dispersed?
e. Today’s extremely slow decay of 238U (with a half-life of 4.5-billion years) means that its daughters, granddaughters, etc. today form slowly. Were these microscopic specks the favored resting places for 218Po for billions of years, or did the decay rate of 238U somehow spike just before all that hot water flowed? Remember, 218Po decays today with a half-life of only 3.1 minutes.
f. Why are isolated polonium halos associated with parallel and aligned myrmekite that resembles tiny ant tunnels?
Answers, based on the hydroplate theory, will soon be given.
Elliptical Halos. Robert Gentry made several major discoveries concerning radiohalos, such as elliptical halos in coalified wood from the Rocky Mountains. In one case, he found a spherical 210Po halo superimposed on an elliptical 210Po halo. Apparently, a spherical 210Po halo was forming, but then was suddenly compressed by about 40% into an elliptical shape. Then, the partially depleted 210Po (whose half-life is 138 days) finished its decay, forming the spherical halo.76
Explosive Expansion. Mineralogists have found, at many places on earth, radial stress fractures surrounding certain minerals that experienced extensive alpha decays. Halos were not seen, because billions of decaying radioisotopes were not concentrated at microscopic points. However, alpha decays throughout those minerals destroyed their crystalline structure, causing them to expand by up to 17% in volume.77
Dr. Paul A. Ramdohr, a famous German mineralogist, observed that these surrounding fractures did not occur, as one would expect, along grain boundaries or along planes of weakness. Instead, the fractures occurred in more random patterns around the expanded material. Ramdohr noted that if the expansion had been slow, only a few cracks—all along surfaces of weakness—would be seen. Because the cracks had many orientations, the expansion must have been “explosive.”78 What caused this rapid expansion? [See Figure 206 and then read, "When, Where, How, and Why Did Radioactive Decay Rates Accelerate?" on page 400.]
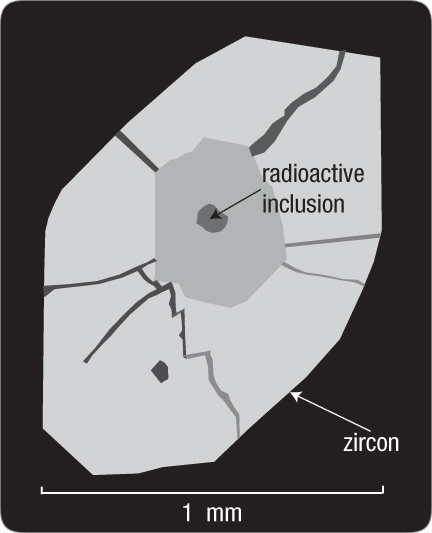
Figure 206: Radial Fractures. Alpha decays within this inclusion caused it to expand significantly, radially fracturing the surrounding zircon that was ten times the diameter of a human hair. These fractures were not along grain boundaries or other surfaces of weakness, as one would expect. Mineralogist Paul Ramdohr concluded that the expansion was explosive. To see why it was explosive, see "When, Where, How, and Why Did Radioactive Decay Rates Accelerate?" on page 400.
Pegmatites. Pegmatites are rocks with large crystals, typically one inch to several feet in size. Pegmatites appear to have crystallized from hot, watery mixtures containing some chemical components of nearby granite. These mixtures penetrated large, open fractures in the granite where they slowly cooled and solidified. What Herculean force produced the fractures? Often, the granite is part of a huge block, with a top surface area of at least 100 square kilometers (40 square miles), called a batholith. Batholiths are typically granite regions that have pushed up into the overlying, layered sediments, somehow removing the layers they replaced. How was room made for the upthrust granite? Geologists call this “the room problem.”79
This understanding of batholiths and pegmatites is based primarily on what is seen today. (In other words, we are trying to reason only from the effect we see back to its cause.) A clearer picture of how and when they formed—and what other major events were happening on earth—will become apparent when we also reason in the opposite direction: from cause to effect. Predictions are also possible when one can reason from cause to effect. Generally, geology looks backward and physics looks forward. We will do both and will not be satisfied until a detailed picture emerges that is consistent from both vantage points. This will help bring into sharp focus “the origin of earth’s radioactivity.”
Theories for the Origin of Earth’s Radioactivity
The Hydroplate Theory. In the centuries before the flood, supercritical water (SCW) in the subterranean chamber steadily dissolved the more soluble minerals in the rock directly above and below the chamber. [Pages 124–125 explain SCW and its extreme dissolving ability.] Thin spongelike channels, filled with high-pressure SCW, steadily grew up into the increasingly porous chamber roof and down into the chamber floor.
The flood began when pressure increases from tidal pumping in the subterranean chamber ruptured the weakening granite crust. As water escaped violently upward through the globe-encircling rupture, pillars had to support more of the crust’s weight, because the subterranean water supported less. Pillars were tapered downward like icicles, so they crushed in stages, beginning at their tips. With each collapse and with each water-hammer cycle, the crust fluttered like a flag held horizontally in a strong wind. Each downward “flutter” rippled through the earth’s crust and powerfully slammed what remained of pillars against the subterranean chamber floor. [See “Water Hammers and Flutter Produced Gigantic Waves” on page 199.]
For weeks, compression-tension cycles within both the fluttering crust and pounding pillars generated piezoelectric voltages that easily reached granite’s breakdown voltage.80 Therefore, powerful electrical currents discharged within the crust repeatedly, along complex paths of least electrical resistance. [See Figures 207–210.]
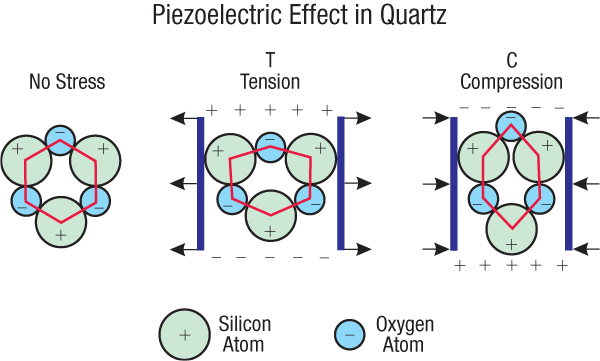
Figure 207: Piezoelectric Effect. Piezo [pea-A-zo] is derived from the Greek “to squeeze” or “to press.” Piezoelectricity is sometimes called pressure electricity. When a nonsymmetric, nonconducting crystal, such as quartz (whose structure is shown above in simplified form), is stretched, a small voltage is generated between opposite faces of the crystal. When the tension (T) changes to compression (C), the voltage changes sign. As the temperature of quartz rises, it deforms more easily, producing a stronger piezoelectric effect. However, once the temperature reaches about 1,063°F (573°C), the piezoelectric effect disappears.81
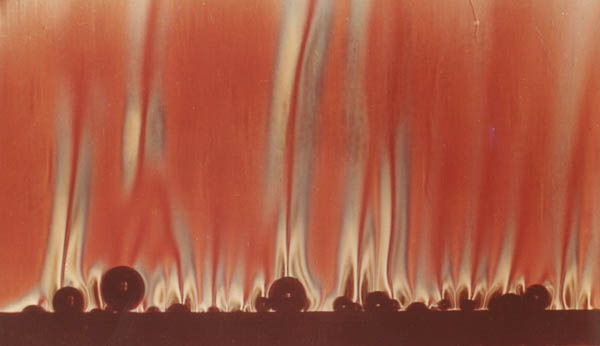
Quartz, a common mineral in the earth’s crust, is piezoelectric. (Granite contains about 27% quartz by volume.) Most nonconducting minerals are symmetric, but if they contain defects, they are to some degree nonsymmetric and therefore are also piezoelectric. If the myriad of piezoelectric crystals throughout the 60-mile-thick granite crust were partially aligned and cyclically and powerfully stretched and compressed, huge voltages and electric fields would rapidly build up and collapse with each flutter half-cycle. If those fields reached about 9 × 10 6 volts per meter, electrical resistances within the granite would break down, producing sudden discharges—electrical surges (a plasma) similar to lightning. [See Figures 199 and 209.] Even during some large earthquakes today, this piezoelectric effect in granite generates powerful electrical activity and hundreds of millions of volts.4 [See “Earthquakes and Electricity” on page 393.]
Granite pillars, explained on page 477 and in Figure 56 on page 127, were formed in the subterranean water, in part, by an extrusion process. Therefore, piezoelectric crystals in the pillars would have had a preferred orientation. Also, before the flood, tidal pumping in the subterranean water compressed and stretched the pillars and crust twice a day. Centuries of this “kneading action” plus “voltage cycling”—twice a day—would align these crystals even more (a process called poling ), just as adjacent bar magnets become aligned when cyclically magnetized. [See Figure 210.] Each piezoelectric crystal acted like a tiny battery—one among trillions upon trillions. So, as the flood began, the piezoelectric effect within pounding pillars and fluttering granite hydroplates generated immense voltages and electric fields. Each quartz crystal’s effective electrical field was multiplied by about 7.4 by the reinforcing electrical field’s of the myriad of nearby quartz crystals.80
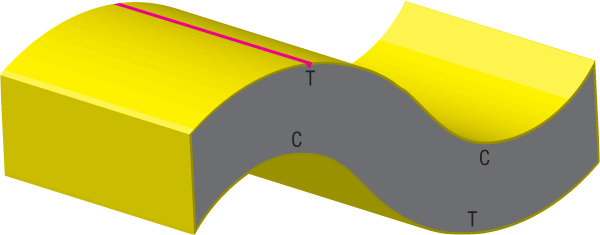
Figure 208: Fluttering Crust. Many of us have seen films showing earth’s undulating crust during earthquakes. Imagine how magnified those waves would become if the crust, instead of resting on solid rock, were resting on a thick layer of unusually compressible water—SCW. Then, imagine how high those waves in the earth’s crust would become if the “ocean” of water below the crust were flowing horizontally with great force and momentum. The crust’s vast area—the surface of the earth (200,000,000 square miles)—gave the relatively thin crust great flexibility during the first few weeks of the flood. As the subterranean waters escaped, the crust flapped, like a large flag held horizontally in a strong wind.
Flutter began as the fountains of the great deep erupted. [See “Water Hammers and Flutter Produced Gigantic Waves” on page 199.] Each time the crust arched downward into the escaping subterranean water, the powerful horizontal flow slammed into the dipping portion of the crust, creating a water hammer that then lifted that part of the crust. Waves rippled through the entire crust at the natural frequencies of the crust, multiplying and reinforcing waves and increasing their amplitudes.
Grab a phone book with both hands and arch it upward. The top cover is in tension, and the bottom cover is in compression. Similarly, rock in the fluttering crust, shown above, would alternate between tension (T) and compression (C). As explained in Figure 207, huge cyclic voltages would build up and suddenly discharge within the granite crust, because granite contains so much quartz, a piezoelectric mineral. Once granite’s breakdown voltage was reached, electrical current—similar to bolts of lightning—would discharge vertically within the crust. Pillars (not shown) at the base of the crust would become giant electrodes. With each cycle of the fluttering crust, current surged through the lower crust, which was honeycombed with tiny pockets of salty (electrically conducting) subterranean water.
Electrons flowing through solids, liquids, or gases are decelerated and deflected by electrical charges in the atoms encountered. These decelerations, if energetic enough, release bremsstrahlung (BREM-stra-lung) radiation which vibrates other nuclei and releases some of their neutrons.
Neutrons will be produced in any material struck by the electron beam or bremsstrahlung beam above threshold energies that vary from 10–19 MeV for light nuclei and 4–6 MeV for heavy nuclei.82
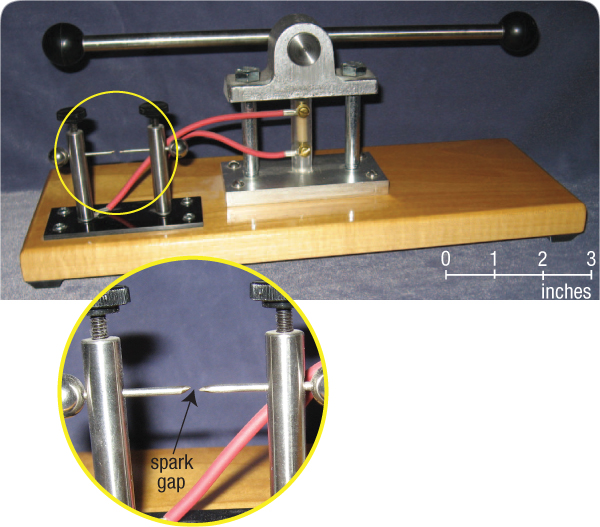
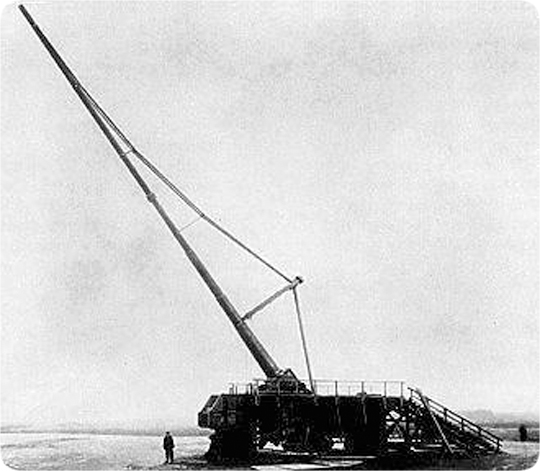
Figure 209: Piezoelectric Demonstration. When I rotate the horizontal bar of this device, a tiny piezoelectric crystal (quartz) is compressed in the vertical column just below the bar’s pivot point. The red cables apply the generated voltage across the two vertical posts mounted on the black, nonconducting platform. Once the increasing voltage reaches about 4,000 volts, a spark (a plasma) jumps the gap shown in the circular inset. When the horizontal bar is rotated in the opposite direction, the stress on the quartz crystal is reversed, so a spark jumps in the opposite direction.
In this device, a tiny quartz crystal and a trivial amount of compression produce 4,000 volts and a small spark. Now consider trillions of times greater compression acting on a myriad of quartz crystals filling 27% of a 60-mile-thick crustal layer. (An “ocean” of subterranean water escaping from below that crust created water hammers, causing the crust to flutter and produce enormous compressive stresses in the crust.) The resulting gigavoltages would produce frightening electrical discharges, not through air, but through rock—and not across a little gap, but throughout the entire crustal layer.
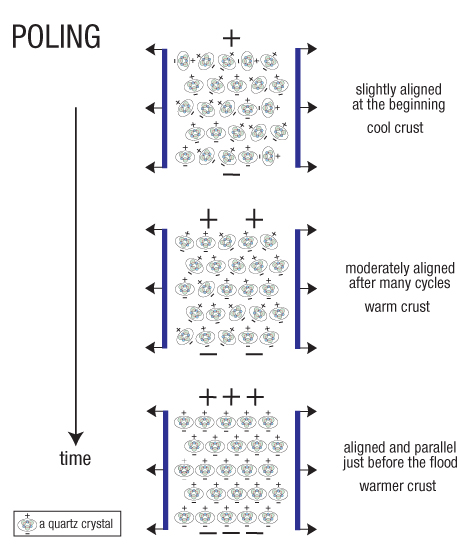
Figure 210: Poling. Poling is an industrial process that steadily aligns piezoelectric crystals so greater voltages can be produced. During the centuries before the flood, tidal stress cycles in the granite crust (tension followed by compression, twice a day), and the voltages and electrical fields they produced, slowly aligned the quartz crystals. (A similar picture, but with arrows and positive and negative signs reversed, could be drawn for the compression half of the cycle.) Over the years, stresses heated the crust to some degree, which accelerated the alignment process. The fact that today so much electrical activity accompanies large earthquakes worldwide shows us that preflood poling was effective. Laboratory tests have also shown that quartz crystals still have a degree of alignment in most quartz-rich rocks.85
At electrical breakdown, the energies in the surging electrons were thousands of times greater than 10–19 MeV, so during the flood, bremsstrahlung radiation released a sea of neutrons throughout the crust.83 Subterranean water absorbed many of these neutrons, converting normal hydrogen (1H) into heavy hydrogen (2H, called deuterium) and normal oxygen (16O) into 18O. Abundant surface water (a huge absorber) protected life.
During the flood, most of this 2H- and 18O-rich subterranean water was swept to the surface where it mixed with surface waters. However, some subterranean water was temporarily trapped within all the mushy mineral deposits, such as salt (NaCl), that had precipitated out of the SCW and collected on the chamber floor years before the flood. Today, those mineral deposits are rich in 2H and 18O.84
The Ukrainian experiments described on page 391 show that a high-energy, Z-pinched beam of electrons inside a solid produces superheavy elements that quickly fission into different elements that are typical of those in earth’s crust. Fusion and fission occur simultaneously, each contributing to the other—and to rapid decay. While we cannot be certain what happens inside nuclei under the extreme and unusual conditions of these experiments, or what happened in the earth’s crust during the flood, here are three possibilities:
a. Electron Capture. Electrons that enter nuclei convert some protons to neutrons. (This occurs frequently, and is called electron capture.)
Also, the dense sea of electrons reduces the mutual repulsion (Coulomb force) between the positively charged nuclei, sometimes bringing them close enough for the strong force to pull them together. Fusion results. Even superheavy nuclei form.
b. Shock Collapse.86 Electrical discharges through the crust vaporize rock along very thin, branching paths “drilled” by gigavolts of electricity through extremely compressed rock. Rock along those paths instantly becomes a high-pressure plasma inside thin rock channels. The shock wave generated by the electrical heating suddenly expands the plasma and the surrounding channel walls, just as a bolt of lightning expands the surrounding air and produces a clap of thunder. As that rock rebounds inward—like a giant, compressed spring that is suddenly released—the rock collapses with enough shock energy to drive (or fuse) nuclei together at various places along the plasma paths. This happens frequently deep in the crust where the rock is already highly compressed.
Superheavy elements quickly form and then fission and decay into such elements as uranium and lead. The heat released propels the plasma and new isotopes along the channels. As the channels contract, flow velocities increase. The charged particles and new elements are transported to sites where minerals are grown, one atom at a time.
c. Z-Pinch. As explained on page 386 and in "Self-Focusing Z-Pinch" on page 407, the path of each electrical charge in a plasma is like a “wire.” All “wires” in a channel are pinched together, but at each instant, pinching forces act only at the points occupied by moving charges, and each force is the sum of the electromagnetic forces produced by all nearby moving charges. Therefore, the closer the “wires,” the greater the self-focusing, pinching force, so the “wires” become even closer, until the strong force merges (fuses) nuclei.
Of these three possible mechanisms, c has the most experimental support, primarily with the 21 billion dollar TOKAMAK (a Russian acronym) being jointly developed by the United States, France, Korea, Russia, the European Union, Japan, India, and China. Items a and b should accompany item c.
For centuries before the flood, SCW dissolved the more soluble minerals in the chamber’s ceiling and floor. The resulting spongelike openings were then filled with SCW.During the flood, that pore water provided an enormous surface area for slowing and capturing neutrons and other subatomic particles. Great heat resulted, some becoming earth’s geothermal heat. Simultaneously, electrical discharges “drilled” thin plasma channels within the crust, producing other nuclear reactions and additional heat.
For weeks, all this heat expanded and further pressurized the SCW in the spongelike channels in the lower crust, slowly forcing that water back into the subterranean chamber. Therefore, higher than normal pressures in the subterranean chamber continuously accelerated the escaping subterranean water, much like a water gun. [See Figure 214.] Velocities in the expanding fountains of the great deep reached at least 32 miles per second , thereby launching the material that became comets, asteroids, meteoroids, and TNOs! [See page 320.]
Heat added to SCW raises temperatures only slightly, for three reasons.
1. Liquid quickly evaporates from the surface of the myriad of microscopic droplets floating in the supercritical vapor. We see surface evaporation on a large scale when heat is added to a pan of water simmering on the stove at 212°F (100°C). The water’s temperature does not rise, but great volumes of vapor are produced.
2. As more heat was added to the escaping SCW, the fountains accelerated even more. With that greater acceleration came greater expansion and cooling.
Nuclear energy primarily became electrical energy and then kinetic energy. Had the nuclear energy produced heat only, much of the earth would have melted.89 Also remember, quartz piezoelectricity shuts off at about 1,063°F (573°C).
Chemical Evolution Theory. The current evolutionary theory for the formation of chemical elements and radioisotopes evolved from earlier theories. Each began by assuming a big bang and considering what it might produce. Years later, fatal flaws were found.
Initially (in 1946), George Gamow, a key figure in developing the big bang theory, said that during the first few seconds after the universe’s hot expansion began, nuclear reactions produced all the chemical elements.103 Two years later, Gamow retracted that explanation. Few heavy elements could have been produced, because the expansion rate was too great, and the heavier the nuclei became, the more their positive charges would repel each other.106
In 1948, the follow-on theory assumed that a big bang produced only neutrons.107 A free neutron decays in about 10 minutes, becoming a proton, an electron, and a particle (an antineutrino) that can be disregarded in this discussion. Supposedly, protons and neutrons slowly merged to become heavier and heavier elements. Later, that theory was abandoned when it was realized that any nucleus with a total of five or eight nucleons (protons or neutrons) will decay and lose one or more nucleons in about a second or less.108 Simply stated, growing a nucleus by adding one nucleon at a time encounters barriers at 5 and 8 atomic mass units.
The next theory said that a big bang produced only hydrogen. Much later, stars evolved. They fused this hydrogen into helium, which usually has four nucleons (two protons and two neutrons). If three helium nuclei quickly merged, producing a nucleus weighing 12 AMU, these barriers at 5 and 8 AMU could be jumped. This theory was abandoned when calculations showed that the entire process, especially the production of enough helium inside stars, would take too long.
A fourth theory assumed that two helium nuclei and several neutrons might merge when helium-rich stars exploded as supernovas. This theory was abandoned when calculations showed that just to produce the required helium, stars needed to generate much more heat than they could produce in their lifetimes.109
The current evolutionary theory for earth’s radioactivity, first proposed in 1952, has the big bang producing only hydrogen, helium, and a trace of lithium. Inside stars, two helium nuclei sometimes merge briefly (for about 7 × 10-17 of a second—less than a billionth of a ten-millionth of a second). If (and what a big “if” that is!), during this brief instant, a third alpha particle merges with the first two, carbon will be formed. But how that triple-alpha process could happen is a mystery.
But exactly how each of these reactions happens at a fundamental level remains unexplained [because all the colliding positively charged nuclei would repel each other].110
This mechanism has not been verified experimentally or computationally.111 Why then, with no scientific support, is this mechanism taught as if it were a fact? Chemical elements had to form somehow. If they did not “evolve,” how did chemical elements get here? This mechanism, as with all prior guesses that were taught widely and are now rejected, is born out of desperation, because creation, the alternative to chemicals evolving, is unacceptable to many.
Even if this problem did not exist, only chemical elements lighter than 60 AMU could be formed—by adding more protons, neutrons, and alpha particles (but only if stars had somehow formed). Pages 29–37 explain why stars, galaxies, and planets would not form from the debris of a big bang.
Assuming the formation of stars and the highly improbable triple collision of alpha particles at a rapid enough rate, stars “burning” hydrogen for billions of years might theoretically produce the rest of the 26 or so lightest chemical elements. But fusion inside stars must stop when nuclei reach about 26 AMU. How the 68 other naturally-occurring chemical elements (those heavier than iron) were produced is not known.114 Charles Seife explains:
We are all made of starstuff. The big bang created hydrogen, helium, and a little bit of lithium and other light atoms. But everything else—the carbon, oxygen, and other elements that make up animals, plants, and Earth itself—was made by stars. The problem is that physicists aren’t quite sure how stars did it.115
Temperatures hundreds of times greater than those occurring inside stars are needed.116 Exploding stars, called supernovas, release extreme amounts of energy. Therefore, the latest chemical evolution theory assumes that all the heavier chemical elements are produced by supernovas—and then expelled into the vacuum of space. By this thinking, radioactive atoms have been present throughout the earth since it, the Sun, and the rest of the solar system evolved from scattered supernova debris.
[Response: Observations117 and computer simulations118 do not support this idea that supernovas produced all the heavy chemical elements. The extreme explosive power of supernovas should easily scatter and fragment nuclei, not drive nuclei together. Remember, nuclei heavier than iron are so large that the strong force can barely hold on to their outer protons. Also, the theoretical understanding of how stars and the solar system formed is seriously flawed. See pages 29–37.]