Below is the online edition of In the Beginning: Compelling Evidence for Creation and the Flood,
by Dr. Walt Brown. Copyright © Center for Scientific Creation. All rights reserved.
Click here to order the hardbound 8th edition (2008) and other materials.
The Hydroplate Theory: Key Assumptions
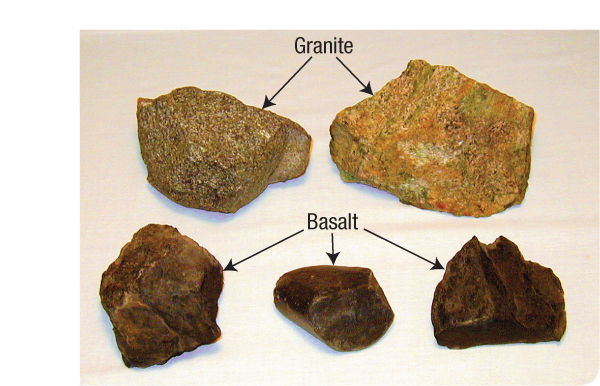
Figure 54: Granite and Basalt. Granite, the primary continental rock, has a grayish-to-pinkish color. Coarse grains of quartz, which have a glassy luster, occupy about 27% of granite’s volume. Basalt, the most common rock beneath oceans today, is solidified lava—a dark, fine-grained rock. The hydroplate theory assumes that before the flood, granite was above the subterranean water and the mantle was below. As you will see, during and after the flood, molten basalt spilled out onto the chamber floor, so most ocean floors today are paved with basalt.
Starting assumptions, as explained above, are always required to explain ancient, unrepeatable events. The hydroplate theory has one major and two minor starting assumptions. All else follows from them and the laws of physics. Proposed explanations for past events always have some initial conditions. Usually they are not mentioned.
Major Assumption: Subterranean Water. About half the water now in the oceans was once in interconnected chambers, 60 miles below the entire earth’s surface. At thousands of locations, the chamber’s sagging ceiling pressed against the chamber’s floor. These solid contacts will be called pillars. The average thickness of the subterranean water was at least 1 mile. Above the subterranean water was a granite crust; beneath that water was earth’s mantle. [See Figure 56.]
Minor Assumption 1: A Global Continent. The earth’s preflood crust encircled the globe. On the crust were deep and shallow seas, and mountains, generally smaller than those of today, but some perhaps 5,000 feet high.
Minor Assumption 2: An Initial Crack. A small initial crack occurred in the earth’s crust. (Several ways this crack could have started will soon be mentioned.) Once a tension crack formed, the high pressures in the chamber and the stress concentrations at each end of the crack would have quickly propagated the crack around the Earth in a great-circle path.
Why does the Mid-Oceanic Ridge also encircle the Earth a great-circle path? The violently escaping subterranean water widened the crack, which removed weight from the chamber floor below. That allowed the chamber floor to bulge upward and form the Mid-Oceanic Ridge. Thus the Mid-Oceanic Ridge also follows a great-circle path.
All 25 major mysteries described earlier, such as major mountain ranges, ice ages, comets, and the Grand Canyon, are consequences of these assumptions. The chain of events that flows naturally from these starting conditions will now be described as an observer might relate those events. The events fall into four phases.
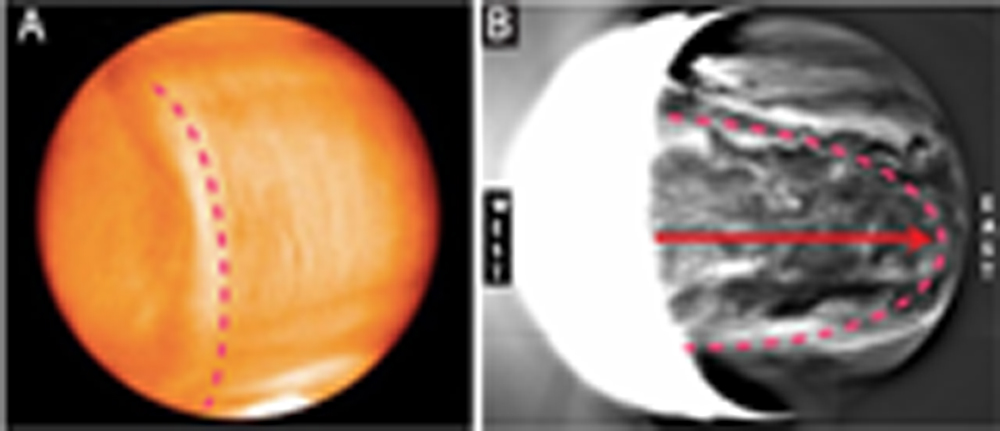
PREDICTION 1: The atmosphere on the day side of Venus will be found to be higher than on the night side.
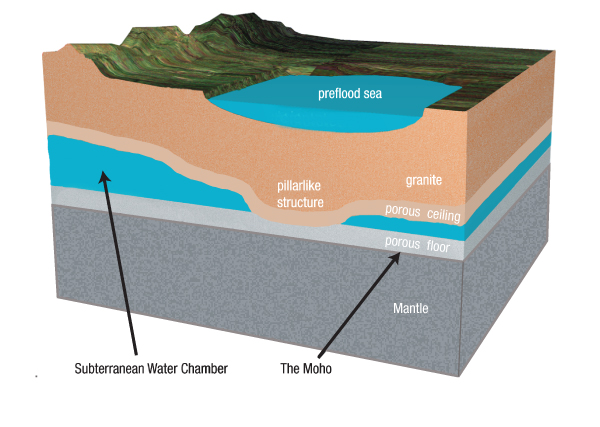
Figure 56: Cross Section of the Preflood Earth. (Not to scale.) Several aspects of the early earth are shown here. The thickness of the subterranean chamber varied, because the chamber’s granite roof sagged and pressed against the chamber floor at locations that will be called pillars. Pillars partially supported the roof. (The confined, high-pressure subterranean water provided most of the support.) Unlike cylindrical pillars we see in buildings, the subterranean pillars were tapered downward. [Pages 477–483 explain how, why, and when pillars formed.]
Supercritical water (SCW) in the subterranean chamber dissolved certain minerals in the chamber’s floor and ceiling—giving that rock a porous, spongelike texture. [SCW is explained on pages 124–125.] High-pressure water filled those voids. The Moho, defined as the sudden transition between slow and fast seismic waves, lies about 3 miles below the chamber floor, marks the bottom of that porous, water-filled layer. This is why seismic waves travel more slowly immediately above the Moho.
Quartz was one of the first minerals to dissolve. This opened tiny grain-size pockets totaling 27% of the volume of granite. Other minerals undoubtedly also dissolved, so the chamber floor and ceiling would have looked like rigid sponges—each a few miles thick. [An interesting ancient writing touches on this. See the quote from The Book of the Cave of Treasures on page 479.] Trapped SCW that filled these tiny pockets remains today. In fact, in 2008, SCW was discovered two miles under the Atlantic floor. Scientists were shocked at finding the first naturally occurring SCW.44 This vast, steady source of superhot water, thick with dissolved minerals, the rare isotope of helium (3He)45, and sometimes hydrocarbons46, is jetting up through the ocean floors as black smokers. [See Figure 57.]
When the flood began, these pockets, a few miles above and below the subterranean chamber, contained much water. To escape to the earth’s surface after the flood, that water had to traverse microscopic, tortuous paths through compressed rock—a very slow process even for a gas or SCW. Black smokers we see today show that small amounts of the subterranean water are still escaping from what was the floor of the subterranean chamber.
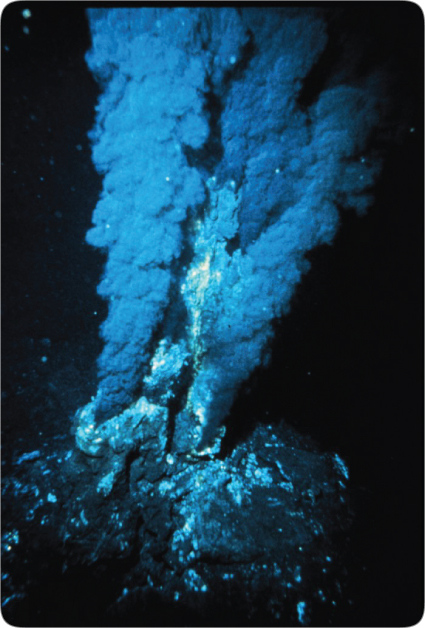
Figure 57: Black Smoker. Black smokers, some as hot as 867°F (464°C), were discovered in 1977 jetting up on a portion of the Mid-Oceanic Ridge in the Pacific. Many other black smokers have since been found along the entire, globe-encircling Mid-Oceanic Ridge, even inside the Arctic Circle and near Antarctica. As hot supercritical water (SCW)44 [explained on pages 124–125], from deep under the ocean floor, shoots up into the frigid ocean, dissolved minerals (and on rare occasions, asphalt ) precipitate out, giving the smoker its black color. SCW can hold vast volumes of dissolved minerals, such as copper, iron, zinc, sulfur, and sometimes hydrocarbons.46 SCW has been produced by man in strong, closed containers, but never before has SCW been seen in its natural state, even around volcanoes.
How do evolutionary geologists explain black smokers? They say water not in a closed container seeps down several miles below the ocean floor—against a powerful and increasing pressure gradient. Magma (molten rock) then heats the water to these incredible temperatures, forcing it back up through the floor. (SCW could not form by such a process, because of the two conditions highlighted in bold above. Uncontained liquid water, heated while slowly seeping downward, would expand, rise, and cool, long before it became supercritical.) Besides, if the evolutionary explanation were true, the surface of the magma body would quickly cool, form a crust, and soon be unable to transfer much heat to the circulating water. (This is why we can walk over lava days after a crust forms. The crust insulates us from the hot lava below.) Obviously, smokers could not be millions of years old, because they are venting so much heat from a finite heat reservoir below. However, black smokers must have been active for many years, because large ecosystems (composed of complex life forms, such as clams and giant tubeworms) have had time to become established around the base of smokers. Figure 56 explains the origin of black smokers.