1. “The discovery that thunderstorms can trigger nuclear reactions provides insight into the physics of atmospheric electricity and unveils a previously unknown natural source of radioactive isotopes on Earth.” Leonid Babich, “Thunderous Nuclear Reactions,” Nature, Vol. 551, 23 November 2017, p. 443.
Those who have read this chapter since it was written in 2009 know that this source of radioactivity has been known and explained since 2009—it was not “previously unknown” in 2017, as quoted above. In fact, piezoelectric activity during the flood is the source of all of Earth’s radioactivity.
u Immediately after lightning crackled through the atmosphere, the detectors would register a burst of gamma rays, followed about 15 minutes later by an extended shower of gamma rays that peaked after 70 minutes and then tapered off with a distinctive 50-minute half-life.” Kim Krieger, “Lightning Strikes and Gammas Follow?” Science, Vol. 304, 2 April 2004, p. 43.
u “It will be shown that the observations of near-ground AGR [atmospheric gamma radiation] following lightning are consistent with the production and subsequent decay of a combination of atmospheric radioisotopes [and new chemical elements] with 10–100 minute half-lives produced via nuclear reactions on the more abundant elements in the atmosphere.” Mark B. Greenfield et al., “Near-Ground Detection of Atmospheric Rays Associated with Lightning,” Journal of Applied Physics, Vol. 93, 1 February 2003, p. 1840.
u A.V. Agafonov et al., “Observation of Neutron Bursts Produced by Laboratory High-Voltage Atmospheric Discharge,” Physical Review Letters, Vol. 111, 12 September 2013, pp. 115003-1 – 115003-5.
These authors consider nuclear fusion as the likely mechanism for these bursts of neutrons. [Thanks to Rick Keane for calling these experiments to my attention on 5 December 2013.]
2. In just 70 billionths of a second, 80 times more electrical current passes through the Z-pinch machine than is consumed in all the world during that same brief time interval. However, that energy is only enough to provide electricity to about five or six houses for an hour. Notice the shortness and intensity of a linear discharge of electrical current.
Similar experiments have been successfully conducted at Texas A & M University.
3. While the physics of the process is well understood, several decades of engineering challenges must be solved before fusion reactors can become an economic reality.
4. For more than a century, stresses in the Earth’s crust have been known to produce powerful voltages and electrical surges. Since 1970, a common explanation for this has been the piezoelectric effect.
“In some parts of the world, earthquakes are often accompanied by ball lighting, stroke lightning and sheet lightning. ... We propose that the piezoelectric effect in the Earth’s crust causes the electrical field. ... In rock with a mean piezoelectric coefficient several percent that of x cut single crystal quartz, and with typical seismic stress changes [of only] 30–300 bars, an earthquake makes an average electrical field of 500–5,000 V cm-1. For distances of the order of half the seismic wavelength, the generated voltage is 5 × 10 7 to 5 × 10 8 V, which is comparable with the voltage responsible for lightning in storms.” David Finkelstein and James Powell, “Earthquake Lightning,” Nature, Vol. 228, 21 November 1970, p. 759.
Other mechanisms may also produce electrical effects from stressed rock, although a clear understanding of those mechanisms is lacking.
All past attempts to identify a physical process that could generate strong currents deep in the ground [by non-piezoelectric mechanisms] have not produced convincing results. Friedemann T. Freund et al., “Electric Currents Streaming Out of Stressed Igneous Rocks—a Step Towards Understanding Pre-Earthquake Low Frequency EM Emissions,” Physics and Chemistry of the Earth, Vol. 31, 2006, p. 390.
Also, other minerals in the crust besides quartz may be piezoelectric. Nevertheless, it is undisputed that stresses in the Earth’s crust will produce powerful voltages and electrical effects. Because the piezoelectric effect is easily explained, well understood, and quantifiable, it will be the mechanism described in this chapter.
5. Briefer, but more intense, compressive stresses and electrical discharges also occurred as the hydroplates crashed near the end of the flood. Because this compression event may be harder to visualize, we will focus primarily on the broader and lengthier events at the beginning of the flood.
6. “No complete theory exists which fully describes the structure and behavior of complex nuclei based solely on a knowledge of the force acting between nucleons [protons and neutrons].” J. S. Lilley, Nuclear Physics (New York: John Wiley & Sons, Ltd, 2001), p. 35.
Various models of the atom are debated. Each explains some things, but each has problems. For example, the popular planetary model visualizes electrons orbiting a nucleus, much as planets orbit the Sun. However, a consequence of Ampere’s Law and Faraday’s Law is that a charged particle, such as an electron, moving in an orbit should radiate energy as electromagnetic waves. Electrons should lose energy and quickly fall into the nucleus. Stated another way:
The “planetary” model assumed that light, negatively charged electrons orbit a heavy, positively charged nucleus. The problem with this model was that the electrons would be constantly accelerating and should radiate energy as electromagnetic waves, causing the atom to collapse. Ibid., p. 4.
Because this does not happen, either electrons do not orbit nuclei, or the above laws must be modified.
Contrary to popular belief, atoms and their components (protons, neutrons, electrons, etc.) are not spheres or mathematical points. This is another example of how we sometimes unknowingly distort reality in order to simplify. Actually, the nuclei of some heavy elements are pear-shaped.
7. Six of the 94 naturally occurring chemical elements have no stable isotopes. Four of the six—Technetium (43), Promethium (61), Astatine (85), and Francium (87)—are formed by cosmic rays and nuclear tests, but soon disappear. Two—Neptunium (93) and Plutonium (94)—are produced by the absorption of neutrons released by the fission of other isotopes. (Atomic numbers—the number of protons in the element’s nucleus—are in blue italics above.) All elements above bismuth (83) are unstable and undergo radioactive decay. As of 2017, 118 elements have been observed, some very briefly in experiments.
8. A few will raise some respectable objections. They say that stars, including our Sun, derive their energy by electrical and magnetic phenomena, not by fusing hydrogen into helium. [See Donald E. Scott, The Electric Sky (Portland, Oregon: Mikamar Publishing, 2006).] We will bypass this fascinating possibility, because the electrical explanation does not address the origin of Earth’s radioactivity.
9. When fusion merges two nuclei heavier than 60 AMU, energy is absorbed, so cooling occurs. The Proton-21 Electrodynamics Research Laboratory in the Ukraine is demonstrating this and is producing superheavy elements. [See page 384.] Fluttering hydroplates at the beginning of the flood and the piezoelectric effect produced similar results. This origin of Earth’s radioactivity also accounts for accelerated radioactive decay and corrects the false belief that the Earth is billions of years old.
10. The instability index is an arbitrary formula that maps half-lives of 0 – · years into an easily visualized 100 – 0 scale.
where C = 10-7 years. For example, a radioisotope with a half-life of 10-7 years (or 3 seconds) would have an instability index of 50. That isotope would be represented by a tall, thin bar that rose halfway up the side of the valley of stability. The data used in constructing this figure were taken from Nuclides and Isotopes: Chart of the Nuclides, 16th edition (Schenectady, NY: Knolls Atomic Power Laboratory, 2002) by Edward M. Baum et al.
11. Why does the valley of stability curve? It is a direct result of “the strong force,” described briefly on page 382. For details, consult a good textbook on nuclear physics.
12. In decay, a nucleus is changed spontaneously (that is, by seemingly random processes inside the vibrating nucleus). Usually a tiny subatomic particle leaves (as in alpha, beta, or gamma decay) or enters (as in electron capture). In fission, a very large nucleus splits into two large nuclei. A wide range of products are possible. Fissions occur in two ways. Either the large nucleus splits after being bombarded by another particle, such as a neutron, or the nucleus splits spontaneously, without bombardment. Spontaneous fissions are considered decays, but most decays are not fissions. Nor are decays nuclear reactions. A nuclear reaction occurs when a nucleus is changed by bombardment. A Z-pinch is a type of nuclear reaction in which increasing magnetic forces squeeze two nuclei so close that the strong force merges them.
Some isotopes, such as 238U, can change in multiple ways: by alpha decay or by fissioning (spontaneously or by bombardment). When 238U fissions spontaneously, it releases four times more energy than when it decays all the way to lead by emitting eight alpha particles and six beta particles. For 238U, alpha decays are 1.8 million times more frequent than spontaneous fissions.
13. “In addition to a particle decay, certain heavy mass nuclei have been observed to decay by emitting 12C, 14C, 20O, 24Ne, 28Mg, or 32Si at extremely low rates. This form of decay has been designated ‘Cluster Radioactivity,’ and was first observed in the emission of 14C from 223Ra. Since 1984, Cluster Radioactivity has been observed in 22 nuclides.” Baum et al., p. 31.
u H. J. Rose and G. A. Jones, “A New Kind of Natural Radioactivity,” Nature, Vol. 307, 19 January 1984, p. 245–247.
u The isotopes that are now known to decay by emitting a carbon-14 nucleus (plus other particles) include: francium-221, radium-221, radium-222, radium-223, actinium-223, radium-224, actinium-225, and radium-226.
14. For example, hydrogen-6 has a half-life of 3 × 10-22 seconds, and tellurium-128 has the longest known half-life: 2.2 × 1024 years. Other isotopes may have more extreme decay rates, but their half-lives are more difficult to measure.
15. H. P. Hahn et al., “Survey on the Rate Perturbation of Nuclear Decay,” Radiochimica Acta, Vol. 23, 1976, pp. 23–37.
A few decay rates increase by 0.2% at a static pressure of about 2,000 atmospheres, the pressure existing 4.3 miles below the Earth’s surface. [See G. T. Emery, “Perturbation of Nuclear Decay Rates,” Annual Review of Nuclear Science, Vol. 22, 1972, pp. 165–202.]
In another static experiment, decay rates increased by 1.0% at pressures corresponding to 930-mile depths inside the Earth. [See Lin-gun Liu and Chih-An Huh, “Effect of Pressure on the Decay Rate of 7Be,” Earth and Planetary Science Letters, Vol. 180, 2000, pp. 163–167.] Obviously, static pressures do not significantly accelerate radioactive decay.
16. K. Makariunas et al., “Effect of Chemical Structure on the Radioactive Decay Rate of 71Ge,” Hyperfine Interactions, Vol. 7, March 1979, pp. 201–205.
u T. Ohtsuki et al., “Enhanced Electron-Capture Decay Rate of 7Be Encapsulated in C60 Cages,” Physical Review Letters, Vol. 93, 10 September 2004, pp. 112501-1 – 112501-4.
17. Richard A. Kerr, “Tweaking the Clock of Radioactive Decay,” Science, Vol. 286, 29 October 1999, p. 882.
18. “The rhenium-187 aeon [billion-year] clock is an example which brings to light—in a rather spectacular manner—the influence of the atomic charge state [electrical charge] on nuclear and astrophysical properties. It has long been recognized that the number and configuration of electrons bound in the atom can significantly alter beta decay lifetimes. However, none of these effects could be investigated until very recently, while only [electrically] neutral atoms were available in the laboratories.” Fritz Bosch, “Setting a Cosmic Clock with Highly Charged Ions,” Physica Scripta, Vol. T80, 1999, p. 34.
u “... a half-life of 32.9 ± 2.0 yr for bare 187Re nuclei could be determined, to be compared with 42 Gyr for neutral 187Re atoms.” Fritz Bosch et al., “Observation of Bound-State b- Decay of Fully Ionized 187Re,” Physical Review Letters, Vol. 77, 23 December 1996, p. 5190.
19. “Unexplained periodic fluctuations in the decay rates of 32Si and 226Ra have been reported by groups at Brookhaven National Laboratory ( 32Si) and at the Physikalisch-Technische Bundesanstalt in Germany ( 226Ra). We show from an analysis of the raw data in these experiments that the observed fluctuations are strongly correlated in time, not only with each other, but also with the distance between the Earth and the Sun.” Jere H. Jenkins et al., “Evidence for Correlations Between Nuclear Decay Rates and Earth-Sun Distance,” arXiv:0808.3283v1 [astro-ph], 25 August 2008, p. 1.
u Davide Castelvecchi, “Half-life (More or Less),” Science News, Vol. 174, 22 November 2008, pp. 20-22.
u “Proximity to the sun seemed to influence radioactivity, and violent activity on the sun could also increase or decrease decay rates.” Corey S. Powell, “Beware: Superflare,” Discover, March 2013, p. 69.
20. Neutrinos are extremely light subatomic particles that travel at nearly the speed of light, carry no electrical charge, and have great ability to pass through matter (without harm). Trillions of neutrinos from the Sun pass harmlessly through each person on Earth every second!
21. See United States Patent 5076971, “Method for Enhancing Alpha Decay in Radioactive Materials,” awarded on 28 August 1989 to William A. Barker. Assignee: Altran Corporation (Sunnyvale, California).
22. Z-pinch (or a self-focusing plasma flow) occurs only if the current exceeds a critical threshold.
Streams of fast electrons which can accumulate positive ions in sufficient quantity to have a linear density of positives about equal to the linear density of electrons, along the stream, become magnetically self-focussing when the current exceeds a value which can be calculated from the initial stream conditions. Willard H. Bennett, “Magnetically Self-Focussing Streams,” Physical Review, Vol. 45, June 1934, p. 890.
That electrical current, according to Bennett [p. 896], turns out to be very small when the voltage is extremely large, as it would be for fluttering hydroplates. That current is
where T is in kelvins and V is in volts. If the plasma’s temperature, T, is 10,000 K and the voltage, V, is 40,000 × 10 6 volts (as explained in Figure 222), then the current required for a Z-pinch is 0.0001 amp—a trivial amount.
With such high voltages, electron velocities become relativistic (become a large fraction of the speed of light). Indeed, One of the key components in the Ukrainian experiments is a relativistic electron beam.
23. “... the nuclei of elements Li, Be, and B are easily destroyed in thermonuclear reactions due to the insufficiently high binding energy.” Adamenko et al., p. 458.
u “Specifically, the rare and fragile light nuclei Lithium, Beryllium and Boron are not generated in the normal course of stellar nucleosynthesis (except 7Li) and are, in fact, destroyed in stellar interiors.” E. Vangioni-Flam and M. Cassé, “Cosmic Lithium-Beryllium-Boron Story,” Astrophysics and Space Science, Vol. 265, 1999, p. 77.
u “Thus the net result is always to convert these elements [deuterium, Li, Be, and B] into helium through proton bombardment, and the rates of the reactions are such that in all conditions before a star evolves off the main sequence all of the deuterium, lithium, beryllium, and boron in the volume which contains the vast majority of the mass will be destroyed.” E. Margaret Burbidge et al., “Synthesis of the Elements in Stars,” Reviews of Modern Physics, Vol. 29, October 1957, p. 618.
24. One might wonder how a star composed of only neutrons could exist if neutrons must be surrounded by protons and electrons to be stable. Yes, neutrons at the surface of a neutron star will tend to decay into a proton, electron, and an antineutrino, but the extreme gravity of a neutron star would probably prevent electrons from permanently escaping from neutrons. [See Lloyd Earnest Busch, “The Paradox of Neutron Decay in Neutron Stars,” Journal of Theoretics, Vol. 5, No. 2, 2003, pp. 10–11.]
25. Paul Giem, “Carbon-14 Content of Fossil Carbon,” Origins, Vol. 51, 2001, pp. 6–30.
u John R. Baumgardner et al., “Measurable 14C in Fossilized Organic Materials,” Proceedings of the Fifth International Conference on Creationism (Pittsburgh, Pennsylvania: Creation Science Fellowship, Inc., 2003), pp. 127–142.
26. Melvin A. Cook, Prehistory and Earth Models (London: Max Parrish, 1966), pp. 66 –67.
27. “The K-Ar method, which is based on the decay of 40K to 40Ar, is probably the most commonly used radiometric dating technique available to geologists.” G. Brent Dalrymple, The Age of the Earth (Stanford, California: Stanford University Press, 1991), p. 90.
28. “This amount of 40Ar is greater by three orders of magnitude than would be expected for a chondritic abundance of potassium in Enceladus’ rock fraction, thus requiring both an efficient mechanism for the escape of 40Ar from the rock component and a mechanism for concentrating it.” J. H. Waite Jr. et al., “Liquid Water on Enceladus from Observations of Ammonia and 40Ar in the Plume,” Nature, Vol. 460, 23 July 2009, p. 488.
29. “The D/H ratio [on Enceladus] is close to the cometary value of 3 × 10-4, nearly twice the terrestrial ocean water value (1.56 × 10-4 ), and more than ten times the value of the D/H ratio in the protosolar nebula (2.1 × 10-5 ).” Ibid.
30. Cook, pp. 66–67.
u “... almost all of the 40Ar and 4He were produced in the Earth.” Frank D. Stacey, Physics of the Earth, 3rd edition (Brisbane, Australia: Brookfield Press, 1992), p. 63.
31. Stanislav Adamenko et al., Controlled Nucleosynthesis: Breakthroughs in Experiment and Theory (Dordrecht, The Netherlands, Springer Verlag, 2007), pp. 1–773.
Those who wish to critically study the claims of Adamenko and his laboratory should carefully examine the evidence detailed in his book. One review of the book can be found at
www.newenergytimes.com/v2/books/Reviews/AdamenkoByDolan.pdf
u “We present results of experiments using a pulsed power facility to induce collective nuclear interactions producing stable nuclei of virtually every element in the periodic table.” Stanislav Adamenko et al., “Exploring New Frontiers in the Pulsed Power Laboratory: Recent Progress,” Results in Physics, Vol. 5, 2015, p. 62.
32. “The products released from the central area of the target [that was] destroyed by an extremely powerful explosion from inside in every case of the successful operation of the coherent beam driver created in the Electrodynamics Laboratory ‘Proton-21,’ with the total energy reserve of 100 to 300 J, contain significant quantities (the integral quantity being up to 10-4 g and more) of all known chemical elements, including the rarest ones.” [emphasis in original] Adamenko et al., p. 49.
In other words, an extremely powerful, but tiny, Z-pinch-induced explosion occurred inside various targets, each consisting of a single chemical element. All experiments combined have produced at least 10 -4 gram of every common chemical element.
u In these revolutionary experiments, the isotope ratios for a particular chemical element resembled those found today for natural isotopes. However, those ratios were different enough to show that they were not natural isotopes that somehow contaminated the electrode or experiment.
33. Stanislav Adamenko, “The New Fusion,” ExtraOrdinary Technology, Vol. 4, October-December, 2006, p. 6.
34. “The number of formed superheavy nuclei increases when a target made of heavy atoms (e.g., Pb) is used. Most frequently superheavy nuclei with A=271, 272, 330, 341, 343, 394, 433 are found. The same superheavy nuclei were found in the same samples when repeated measurements were made at intervals of a few months.” Adamenko et al., “Full-Range Nucleosynthesis in the Laboratory,” Infinite Energy, Issue 54, 2004, p. 4.
35. “The energy of a coherent driver [the electron beam] is equal to only a small part of the total energy released in the process of transformation of nuclei of the target [electrode] into nuclei of the synthesized isotopes. In fact, in the zone of the self-organized collapse, we are faced with the process of a distinctive “cold repacking” of nucleons which initially belonged to nuclei of the target. This process terminates in the final configuration which corresponds to newly synthesized isotopes. ... the process is adiabatic.” Ibid., p. 3.
36. Stanislav Adamenko, “Results of Experiments on Collective Nuclear Reactions in Superdense Substance,” Proton-21 Electrodynamics Laboratory, 2004, pp. 1–26. For details see
www.proton21.com.ua/articles/Booklet_en.pdf.
u “Frequently Asked Questions,” Proton-21 Electrodynamics Laboratory. See: www.proton21.com.ua/faq_en.html.
u Stanislav Adamenko, Personal communication, 13 April 2010.
37. “The first 700 million years of Earth’s 4.5-billion-year existence are known as the Hadean period, after Hades, or, to shed the ancient Greek name, Hell. That name seemed to fit with the common perception that the young Earth was a hot, dry, desolate landscape interspersed with seas of magma and inhospitable for life.” Kenneth Chang, The New York Times, 2 December 2008, p. D1.
38. Michelle Hopkins et al., “Low Heat Flow Inferred from > 4 Gyr Zircons Suggest Hadean Plate Boundary Interactions,” Nature, Vol. 456, 27 November 2008, pp. 493–496.
39. “The origin of the carbon and the nature of the carbon reservoir, as well as the process by which microdiamonds can be incorporated in zircon together with ‘granitic’ inclusions, present problems fundamental to understanding processes active in the early history of the Earth. ... The observed large variations in [carbon isotope ratios] inclusions hosted in the same zircon grain suggest that the carbon inclusions formed from different material and/or under different geological conditions before they were eventually included in the zircon. ... Therefore, the simplest explanation, and the one which is supported by most observations, is that the diamond formation must pre-date zircon crystallization and, most probably, is not related to zircon formation.” Alexander A. Nemchin et al., “A Light Carbon Reservoir Recorded in Zircon-Hosted Diamond from the Jack Hills,” Nature, Vol. 454, 3 July 2008, pp. 92–93.
40. “In fact, considering the Precambrian age of the granite cores [containing zircons], our results show an almost phenomenal amount of He has been retained at higher temperatures, and the reason for this certainly needs further investigation ...” Robert V. Gentry et al., “Differential Helium Retention in Zircons,” Geophysical Research Letters, Vol. 9, October 1982, p. 1130.
u D. Russell Humphreys, “Young Helium Diffusion Age of Zircons Supports Accelerated Nuclear Decay,” Radioisotopes and the Age of the Earth, editors Larry Vardiman et al. (El Cajon, California: Institute for Creation Research, 2005), pp. 25–100.
41. How is 3He produced? Nuclear reactions first produce 3H (tritium), either as a rare fission product, or in one of the following ways: (Remember free neutrons are rare and have a half-life of only 14.7 minutes.)
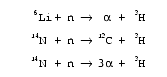
Then, a beta decay (with a half-life, today, of 12.32 years) converts 3H into 3He. [See L. T. Aldrich and Alfred O. Nier, “The Occurrence of He3 in Natural Sources of Helium,” Physical Review, Vol. 74, 1 December 1948, pp. 1590–1594.]
42. “But the questions of how gas from the solar nebula was trapped in the solid parts of growing planets, and how the gas was preserved through early accretionary events, will certainly test our models of accretion.” Chris J. Ballentine, “A Dash of Deep Nebula on the Rocks,” Nature, Vol. 486, 7 June 2012, p. 41.
43. “They found [in Siberian flood basalts] that the ratio of helium 3 to helium 4 was not just 8 times greater than the atmospheric ratio, as it is at midocean ridges, but 13 times greater.” Marc Zabludoff, “Breakthroughs, Geology,” Discover, Vol. 16, December 1995, p. 122.
u The ratio of 3He to 4He varies widely in rocks near oceanic trenches, among deposits of natural gas, and within the Hawaiian Islands.
44. “... the location or process that could prevent such a deep reservoir [of 3He] from mixing into the convecting mantle and disappearing completely have remained enigmatic.” Ballentine, p. 41.
45. H. S. Carslaw and J. C. Jaeger, Conduction of Heat in Solids, 2nd edition (Oxford: At the Clarendon Press, 1959), p. 87.
u R. J. Strutt (son of the famous Lord Rayleigh who made many scientific discoveries, including the discovery of argon) first explained this in 1906, ten years after Henri Becquerel discovered radioactivity. Strutt measured radioactivity in various rocks and found that granite contained more than enough radioactivity to explain all geothermal heat. He concluded that “Earth’s radioactivity was confined to the crust, a few tens of kilometers thick.” [See Stacey, Physics of the Earth, 3rd edition (1992), p. 45.]
u Each year on average, radioactive decay releases W calories of heat per cubic centimeter of granite, and S calories of heat escape into the atmosphere from each square centimeter of continental (granitic) crust. A layer of granite only S/W thick would account for all this heat, if steady state has been reached. Here are some reported values of W and S:
As explained on pages 153–192, other heat sources are generating heat within the Earth, so these thicknesses of granite would be even thinner. The granite crust is generally estimated to be at least 50 km (30 miles) thick. Therefore, steady state has not been reached. In other words, radioactivity is concentrated in the crust but has not been there long enough to reach steady state.
u “Surface rocks show traces of radioactive materials, and while the quantities thus found are very minute, the aggregate amount is sufficient, if scattered with this density throughout the earth, to supply, many times over, the present yearly loss of heat. In fact, so much heat could be developed in this way that it has been practically necessary to make the assumption that the radioactive materials are limited in occurrence to a surface shell only a few kilometers in thickness.” Leonard R. Ingersoll et al., Heat Conduction: With Engineering, Geological and Other Applications, revised edition (Madison, Wisconsin: University of Wisconsin Press, 1954), p. 102.
u “Uranium, thorium and potassium are the main elements contributing to natural terrestrial radioactivity. ... All three of the radioactive elements are strongly partitioned into the continental crust.” J. A. Plant and A. D. Saunders, “The Radioactive Earth,” Radiation Protection Dosimetry, Vol. 68, 1996, p. 25.
46. “... the molten rock oozing from midocean ridges lacks much of the uranium, thorium, and other trace elements that spew from some aboveground volcanoes.” Sid Perkins, “New Mantle Model Gets the Water Out,” Science News, Vol. 164, 13 September 2003, p. 174.
47. “... 90% of uranium and thorium are concentrated in the continents. In general, the heat production rate must decrease with depth. Otherwise, surface values would imply zero or negative mantle heat flow.” Dan F. C. Pribnow, “Radiogenic Heat Production in the Upper Third of Continental Crust from KTB,” Geophysical Research Letters, Vol. 24, 1 February 1997, p. 349.
Continents contain much less than 1% of the Earth’s mass (actually 0.35%), so why do they have 90% of Earth’s uranium and thorium?
48. “The measured temperature gradient of 27.5 K km-1 in the upper 9.1 km [5.7 miles] cannot continue to the Moho, otherwise a boundary condition derived from seismic interpretations is violated.” Ibid., pp. 351–352.
In other words, the rocks directly below the Moho would have melted—an easily detected condition. Decades ago, students were taught that the mantle was a liquid. Even today, some textbooks make this erroneous claim. If the mantle had only a thin, continuous shell of liquid at any depth, certain seismic waves (shear waves, also called secondary waves) could not pass through that shell. However, seismometers all over the world measure those waves daily.
49. Robert F. Roy et al., “Heat Generation of Plutonic Rocks and Continental Heat Flow Provinces,” Earth and Planetary Science Letters, Vol. 5, 1968, pp. 1–12.
50. For example, did you know that a person’s foot size correlates with writing ability? Does this mean that the bigger your feet, the better you write? No. It means that babies don’t write well.
Although correlations may suggest a cause and effect relationship, they do not demonstrate cause and effect. For that, mechanisms and experimental results are needed.
51. So far, 16 zones have been discovered; some are connected.
52. If 100 neutrons were somehow produced in the first generation, and x neutrons were produced in the second generation, the reactor’s efficiency would be x percent. If
the total number of neutrons produced would be
If k = 0.6, a total of 250 neutrons would be produced for every 100 initial neutrons. With an efficiency of 99%, 10,000 neutrons would be produced. If a trillion neutrons were produced in the first generation, and the efficiency were 99%, a total of 100 trillion neutrons would be produced.
53. “Reactors 7 to 9 [discovered in 1978] ... appear as small uranium-rich pockets where the core of the reactor is always very thin (a few centimeters) ... .” F. Gauthier-Lafaye et al., “Natural Fission Reactors in the Franceville Basin, Gabon: A Review of the Conditions and Results of a ‘Critical Event’ in a Geologic System,” Geochimica et Cosmochimica Acta, Vol. 60, No. 23, 1996, p. 4838.
54. “The anomalous behavior at the reactor zone borders should be further investigated to determine if it is a general phenomenon capable of a common explanation such as the ‘reflux’ hypothesis presented in this paper.” G. A. Cowan et al., “Some United States Studies of the Oklo Phenomenon,” The Oklo Phenomenon (Vienna: Vienna International Atomic Energy Agency, 1975), p. 355.
In a later paper, Cowan acknowledged that the “reflux hypothesis” did not explain the problem and that “puzzling anomalies” remained at the borders. [See George A. Cowan, “A Natural Fission Reactor,” Scientific American, Vol. 235, July 1976, p. 44.]
55. S. Hishita and A. Masuda, “Thousandfold Variation in 235U/ 238U Ratios Observed in a Uranium Sample from Oklo,” Naturwissenschaften, Vol. 74, May 1987, pp. 241–242.
56. A. A. Harms, “Reaction Dynamics and 235U/ 238U Ratios for the Oklo Phenomenon,” Naturwissenschaften, Vol. 75, January 1988, pp. 47–49.
57. William R. Corliss has cataloged many books and reports of electrical activity associated with earthquakes. My brief extracts, slightly edited, are taken from his Strange Phenomena (Glen Arm, Maryland: The Sourcebook Project, 1974), Vol. G1, pp. 183–204 and Vol. G2, pp. 135–151.
58. Myron L. Fuller, The New Madrid Earthquake (Washington, D.C.: USGS Bulletin 494, 1912), p. 46.
59. Radiohalos have been found in more than 40 minerals. [See Robert V. Gentry, “Radioactive Halos,” Annual Review of Nuclear Science, Vol. 23, 1973, p. 350.]
60. G. H. Henderson and F. W. Sparks, “A Quantitative Study of Pleochroic Halos, p. 243.
61. Actually, almost all (9,998 out of 10,000) 218Po isotopes decay by emitting an alpha particle. A few emit a beta particle.
62. Robert V. Gentry, Creation’s Tiny Mystery, 2nd edition (Knoxville, Tennessee: Earth Sciences Associates, 1988).
Robert Gentry, in several dozen papers in leading scientific journals, has reported important discoveries concerning these mysteries. He may be the one person most responsible for showing that the Earth’s crust was never molten and, therefore, did not evolve. The importance of Gentry’s work is shown by the intensity of the opposition he has received; yet, many of his opponents admit in published writings that they cannot explain parentless polonium halos. To minimize that major problem, opponents often refer to this as “a tiny mystery.” No, only the halos are tiny; the mystery to evolutionists is great, and the dilemma this presents to those who believe in a 4.5-billion-year-old Earth is even greater.
63. If a billion polonium-218 ( 218Po) atoms had ever been concentrated in a tiny inclusion in dry rock, the heat generated within one half-life (3.1 minutes) would melt an isolated sphere of radius 0.0033 cm. This is 40% larger than the final 218Po halo radius of 0.0023 cm. Since polonium halos never melted, as explained in Endnote 64, we can conclude that a billion 218Po atoms were never concentrated at any tiny inclusion in dry rock at the same time. This includes the time of the rock’s creation. The actual melting would begin at the instant of creation (t=0) and rapidly advance outward from the center to a distance of 0.0033 cm in 3.1 minutes. Assume that a billion 218Po atoms are concentrated in a tiny inclusion. Half would eject an alpha particle within 3.1 minutes—each alpha particle releasing 6.0 MeV of energy. (1 MeV = 3.83 × 10-14 cal) Of those 500,000,000 alpha particles, the first 375,000,000 would raise the sphere’s temperature up to the rock’s melting point. The remaining 125,000,000 alpha particles would melt the entire sphere.
To verify the above statements, the following properties of the rock will be used:
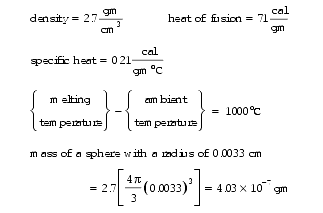
and the following two heat-balance equations can be easily and quickly checked. First, raising the sphere’s temperature to its melting point:
Then, melting the rock:
So why do we see unmelted polonium halos?
i. Each 218Po ion was electrically attracted within minutes to a tiny inclusion after it formed by the decay of 222Rn. [See "Rapid Attraction" on page 348.] With trillions of 222Rn atoms transported in the water flowing through the spongelike channels in the crust, and many 218Po ions simultaneously moving toward their destination, this could have taken days or weeks, enough time for the heat to transfer away as the halo slowly formed.
ii. The halos were cooled by considerable flowing subsurface water and by the “evaporation” of the volatile OH -.
For details, see “Isolated Polonium Halos” on pages 406–408.
64. Gentry conducted tests that confirmed that melting did not occur. [See Robert V. Gentry, “Radiohalos in a Radiochronological and Cosmological Perspective,” Science, Vol. 184, 5 April 1974, pp. 62–66.]
65. Gentry never observed this concentration of halo centers in specific sheets. Personal communication, 7 August 2009.
66. Henderson and Sparks, “A Quantitative Study of Pleochroic Halos, IV, ” Proceedings of the Royal Society of London, Series A, Vol. 173, 1939, pp. 238–249.
u G. H. Henderson, “A Quantitative Study of Pleochroic Halos, V,” Proceedings of the Royal Society of London, Series A, Vol. 173, 1939, pp. 250–263.
67. More specifically, the mine’s intrusions were “calcite vein dikes (rocks containing mostly the mineral calcite and other minerals, such as mica) that are small in length and width and cut metasedimentary rocks which still retain bedding planes.” [See J. Richard Wakefield, “Gentry’s Tiny Mystery,” Creation/Evolution, Vol. 22, Winter 1987–1988, p. 17.]
u Gentry discusses this trip on pages 325–327 of Creation’s Tiny Mystery. Wakefield discusses it in the reference above.
68. “... the existence of polonium halos in the biotite at the Fission and Silver Crater Mines [near Bancroft, Ontario] serves to identify the host ‘vein dikes’ as also being created rocks, ...” Robert V. Gentry, “Response to Wise,” Creation Research Society Quarterly, Vol. 25, March 1989, p. 177.
u “... [Wakefield] implies that certain ‘intrusive,’ crystalline rocks discount a creation origin for those rocks, but the fact is, my creation model includes these among the rock types that were created [as solids].” Robert V. Gentry, “Response to Wakefield’s Remarks,” Creation’s Tiny Mystery, p. 325.
69. Kurt P. Wise, “Radioactive Halos: Geologic Concerns,” Creation Research Society Quarterly, Vol. 25, March 1989, pp. 171–176.
70. Lorence G. Collins, “Polonium Halos and Myrmekite in Pegmatite and Granite,” Expanding Geospheres, Energy and Mass Transfers from Earth’s Interior, editor C. Warren Hunt (Calgary: Polar Publishing Company, 1992), p. 132.
Obviously, Collins overstates his case, because he could not have checked “all of the granites in which Gentry found polonium halos.” Nevertheless, myrmekites were found in many of those granites.
71. Feldspars are a class of minerals that constitute almost 60% of the Earth’s crust. The subgroup, plagioclase feldspars, comes in two varieties: calcium-rich and sodium-rich. Myrmekite contains only the sodium variety. Sodium feldspars form when sodium (Na1+) and silicon (Si4+) replace calcium (Ca2+) and aluminum (Al3+) in calcium feldspars.
An alert reader may wonder (1) where all the calcium went, and (2) what provided the silicon for the replacement. The chapter "The Origin of Limestone" on pages 259–264 answers the first question. Pages 126– 127, which explain the extreme solubility of quartz (SiO2) in supercritical water (SCW), answer the second.
What accounts for the replacement of aluminum (Al) with sodium (Na) in the sodium feldspars? Answer: SCW readily dissolves aluminum (which opened up slots in calcium feldspars). Salt (NaCl) was dissolved in SCW as Na+ and Cl-. The Na+ then entered those slots.
72. “... several ‘puzzles’ that still challenge the geologic profession: ... Why are Po halos in biotite and fluorite associated with myrmekite-bearing granites?” Lorence G. Collins, Hydrothermal Differentiation and Myrmekite—A Clue to Many Geologic Puzzles (Athens, Greece: Theophrastus Publications, S.A., 1988), p. 5.
73. “The Po halos are observed to occur primarily in biotite and fluorite in pegmatites and in biotite in granite in terranes where the granite is myrmekitic.” Ibid., p. 232.
74. “Thus, polonium was deposited in new crystals that grew from voluminous hydrothermal flushing of sheared and fractured, formerly-solid, mafic rock. ... Rapid entry of radon and precipitation of polonium could occur if a gabbro or diorite site were made porous and depressurized by tectonism.” Collins, “Polonium Halos and Myrmekite in Pegmatite and Granite,” pp. 135, 136.
u Collins’ explanation is a more detailed refinement of the explanation by Canadian physicist G. H. Henderson in 1939, one of the earliest radiohalo researchers. [See Endnote 60.] Others have proposed less-successful variations of Henderson’s basic insight or have repackaged Collins’ explanation without proper credit.
75. Collins’ vague explanation lacks specifics and a mechanism.
The creeping rock-movements associated with seismically-active terranes open avenues for radon-bearing water to move into lower-pressured pore space, and to the surface. Collins, “Polonium Halos and Myrmekite in Pegmatite and Granite,” p. 134.
“Creeping”? Why “seismically-active”? Why was there so much “radon-bearing water”? The radon in question, 222Rn, has a half-life of only 3.8 days. What “opened ‘avenues’ inside rock for radon-bearing water” and when? What provided the necessary energy and forces?
76. Photographs of these elliptical halos can be seen in Plate 5 of Gentry’s Radiohalo Catalogue in Creation’s Tiny Mystery.
77. Bryan C. Chakoumakos et al., “Alpha-Decay Induced Fracturing in Zircon: The Transition from the Crystalline to the Metamict State,” Science, Vol. 236, 19 June 1987, pp. 1556–1559.
78. “Fractures pay not the least attention to the cohesion minimums and not even to grain boundaries, where slip would take place so easily under stresses, but evidently occur quite suddenly in the form of an explosive fracture and not a slow expansion. The evidently simultaneous effect on various other constituents including those of rather different hardness and tenacity are proof of the above. The sudden released energy must be enormous in individual cases. The author observed fracture circles about orthite in quartz of about 1 meter diameter in the Iveland district in southern Norway!” Paul A. Ramdohr, “New Observations on Radioactive Halos and Radioactive Fracturing,” Oak Ridge National Laboratory Translation (ORNL-tr-755), 26 August 1965, p. 19.
79. “One of the major problems in determining the origin of batholiths of granite composition is to explain what happened to the country rock [the older rock] that was displaced by the invading magma.” [See Arthur N. Strahler, Physical Geology (New York: Harper & Row, Publishers, 1981), p. 912.]
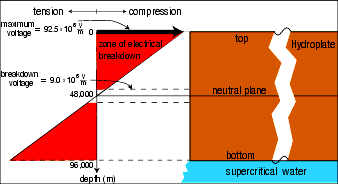
Figure 222: Sea of Neutrons. Piezoelectric voltages were produced by compressive and tensile stresses in the fluttering crust acting on trillions upon trillions of quartz crystals. Because those cyclic stresses varied from a maximum at the top and bottom of the crust to zero at the neutral plane in the middle, the piezoelectric voltages also decrease linearly to zero at the neutral plane. Therefore, the total piezoelectric voltages exceeded the breakdown voltage of 9 × 10 6 V/m throughout almost all of the 60-mile thick hydroplate (shown in red). However, the excess energy gained in accelerating electrons in the top and bottom of the hydroplate produced breakdown throughout the entire crust. This energy of almost
92.5 ×106 × 48,000 × 0.5 = 2.2 ×1012 = 2.2×106 MeV
was many orders of magnitude larger than the 10–19 MeV necessary for bremsstrahlung radiation to release free neutrons. Therefore, a sea of neutrons resulted which produced new isotopes throughout the crust.
u “A second problem involves the great volume [hundreds of cubic miles in some cases] of pre-existing country rock which must be removed to provide space for an invading batholith—the eliminated country rock must be accounted for somehow.” [See W. G. Ernst, Earth Materials (Los Angeles: Prentice-Hall, Inc., 1969), p. 108.]
80. A cyclic load on granite will temporarily produce a cyclic voltage. Normally, free electrons in the Earth will neutralize the voltage in a few seconds. However, for the fluttering crust, supercritical water (SCW), a strong and vast dielectric, electrically insulated the crust from below, so free electrons from the rest of the Earth could not flow up to neutralize the voltage. As cyclic voltages built up and suddenly discharged within the fluttering crust, the electrical charges within the ionized SCW shifted back and forth by induction.
Once the temperature of quartz exceeds about 1,063°F (573°C), its atoms become mobile enough to reorient and neutralize any voltage.
81. Each quartz crystal, when stressed, sets up an electrical field which reinforces the electrical fields of all nearby quartz crystals. Each field’s strength diminishes as the square of the distance from the crystal source, and is also reduced by about 80% by granite’s permittivity (resistance to the electrical field). Nevertheless, so many crystals lie within granite that their three-dimensional integrated effect amounts to 7.4 times that of one quartz crystal alone.
In carrying out this integration, the granite hydroplate was divided into tiny but equal cubic volumes, each containing a quartz crystal. (Quartz crystals occupy about 27% of granite by volume.) Then, the effects of all quartz crystals were summed from 1 to infinity in all three dimensions. This uniformity assumption is conservative, since electrical breakdown will occur on the easiest path, not the harder paths that would exist if the quartz crystals were of identical sizes and uniformly spaced within the granite. Figure 222 shows that the entire hydroplate experienced electrical breakdown and a huge flux of neutrons from bremsstrahlung radiation.
Quartz crystals generate about 0.0625 volt (V) per meter for each N/m2 (newton per square meter) of compression. [See http://en.wikipedia.org/wiki/Piezoelectric.] Granite’s compressive strength is about 2 × 10 8 N/m2. The crushing seen within the granite crust tells us that such compressive stresses have been exceeded in the past, and the observed electrical activity during modern earthquakes shows that breakdown thresholds are even being reached today. [See "Earthquakes and Electricity" on page 387.] Certainly, stresses exceeded this during the compression event that pushed up all of Earth’s major mountain ranges. Therefore, electric fields of at least 92.5 × 10 6 V/m were reached in the extreme top and bottom of each hydroplate.
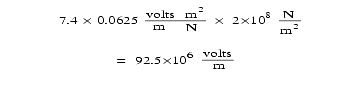
Notice in Figure 222 how this exceeds the breakdown voltage of dry granite: 9 × 10 6 V/m. [See Smithsonian Physical Tables, 9th revised edition (Norwich, N.Y., Knovel, 2003), p. 423.]
The total voltage generated in the fluttering crust is equal to the area of a red triangle in Figure 222 (volts/meter times the half-thickness of the crust in meters). This voltage (and therefore the z-pinching) was orders of magnitude greater than a brief 1-billion volt bolt of lightning is on our low-density atmosphere today. Shock collapse (explained on page 392) also contributed a powerful additional pinch as did the compression event and the pounding pillars.
Rock is weak in tension, so when the top half of a hydroplate was in the tension half of its flutter cycle, these high voltages were not reached near the Earth’s surface (as they were in the compression half cycle). However, in the bottom half of a hydroplate, tension only means that the large compressive stresses due to the weight of the overlying rock were reduced by the amount of tension. Therefore, cyclic changes in stress in the bottom half, during both the tension and compression half cycle, produced these extreme voltages.
Temperature is another important variable. The above properties were measured at room temperatures. As temperatures increase up to the limit of 1,063°F (573°C) mentioned in Endnote 80, the piezoelectric coefficient increases and breakdown voltages decrease—both contributing to more extensive and powerful plasma production.
82. N. E. Ipe, “Radiological Considerations in the Design of Synchrotron Radiation Facilities,” Stanford Linear Accelerator Center, SLAC-PUB-7916, January 1999, p. 6.
83. To see why powerful bremsstrahlung radiation releases neutrons, a review will be helpful. On page 382, we introduced “the strong force” by asking, “Why don’t large nuclei fly apart, since they contain positive charges (protons) which should repel each other?
In addition to the strong force that holds tightly packed protons and neutrons together, neutrons inside a nucleus spread the protons farther apart, thereby reducing their mutual repulsion. That repelling force, like air pressure in a balloon, gives the nucleus a spherical shape if no other force acts upon it. However, if powerful piezoelectric voltages produce electrical surges near these nuclei, the electrons will emit bremsstrahlung radiation as they decelerate. The trillions of cycles per second of alternating positive and negative charges in that radiation will vibrate the protons in the nucleus, so the nucleus takes on a different ellipsoid (or football) shape each cycle. The portions of the nucleus that are farthest from the center of the nucleus (at the tips of the football shape) will more nearly resemble smaller, but neutron-heavy nuclei. Therefore, they quickly expel neutrons.
As explained on page 381 in discussing the valley of stability, small stable nuclei usually have as many neutrons as protons. For example, helium usually has two of each, carbon has three of each, and oxygen has eight of each. For more massive nuclei to be stable more neutrons than protons are needed to spread the protons farther apart and reduce their mutual repulsion. (For example, uranium has 92 protons and most uranium nuclei have 146 neutrons.) Therefore, a powerfully vibrating heavy nucleus distorts into shapes where portions of the nucleus have too many neutrons close together. To be stable at that instant, those portions must expel a few neutrons. This is why the powerful bremsstrahlung radiation during the compression event near the end of the flood released “a sea” of neutrons.
If the neutrons released in each fission produce, on average, exactly one more fission, the concentration of 235U is said to be critical. If more than one fission occurs on average, there is an explosion, as in an atomic bomb.
For the same reason, when a neutron—acting as a bullet— splits (fissions) a uranium-235 (235U) nucleus, the two smaller fragments no longer need as many neutrons, so each typically releases two or three neutrons.
u Electrons accelerated in a plasma by high-energy lasers will produce neutrons, positrons, and fission fragments by bremsstrahlung radiation. [See P. L. Shkolnikov and A. E. Kaplan, “Laser-Induced Particle Production and Nuclear Reactions,” Journal of Nonlinear Optical Physics and Materials, Vol. 6, No. 2, 1997, pp. 161–167.]
84. “The spatial variation in d18O (Fig. 1) can most easily be explained by the upward migration along the flank of the [salt] dome of diagenetically altered waters enriched in heavy oxygen ... .” Jeffrey S. Hanor, “Kilometre-Scale Thermohaline Overturn of Pore Waters in the Louisiana Gulf Coast,” Nature, Vol. 327, 11 June 1987, p. 501.
u “Sulfate ions in saline lakes and brines have oxygen-18 enrichment of from 7 to 23 per mille relative to mean ocean water;” A. Longinelle and H. Craig, “Oxygen-18 Variations in Sulfate Ions in Sea Water and Saline Lakes,” Science, Vol. 156, 7 April 1967, p. 56.
u “Results indicate both higher enrichments of heavier isotopes [of 2H and 18O] and higher chloride concentrations in water samples from salt pans than in water samples from other sources.” H. Chandrasekharan et al., “Deuterium and Oxygen-18 Isotopes on Groundwater Salinization of Adjoining Salt Pans in Porbandar Coast, Gujarat, India,” Hydrochemistry, IAHS Publication No. 244, April 1997, p. 207.
85. “All quartz-rich rocks (quartzites, granites, gneisses, mylonites) did show [statistically significant] piezoelectric effects when stressed.” J. R. Bishop, “Piezoelectric Effects in Quartz-Rich Rocks,” Tectonophysics, Vol. 77, 20 August 1981, p. 297.
u “... frequently in quartzite, the quartz occurs as grains with isometric form but shows a preferential orientation in terms of internal crystal structure, that is, in terms of the axes of crystallization.” E. I. Parkhomenko, Electrical Properties of Rocks (New York: Plenum Press, 1967), p. 6.
86. J. R. Rygg et al., “Dual Nuclear Product Observations of Shock Collapse in Inertial Confinement Fusion,” LLE Review, Vol. 111, pp. 148–153.
87. The photo of this lightning rod can be seen at:
http://en.wikipedia.org/wiki/Plasma_pinch.
After the owner of this photograph gave permission to use his image of the lightning rod, he withdrew permission, because he did not want his photo “used for such nonscientific purposes” as this book. (No one should think that all scientists are unbiased and freely exchange data and information. Some even suppress information.) In three other instances involving different topics, evolutionists denied permission to use photographs for this book, although copyright fees were offered.
88. Bennett, pp. 890–897.
89. The following definitions pertain to this calculation:
mole : the mass of a substance equal to its atomic or molecular weight expressed in grams. For example, a mole of carbon-12 weighs 12 grams. A mole of water (H2O or 1H + 1H +16O) is 18 grams of water.
Avogadro’s number : the number (6.022 × 1023) of atoms or molecules in one mole. For example, 12 grams of carbon contain 6.022 × 1023 carbon atoms.
erg : a unit of energy or work done by a force of 1 dyne acting through a distance of 1 centimeter. For example, a 1-pound brick falling through 1 foot releases 13,600,000 ergs of energy.
MeV : a million electron volts (a small unit of energy). It is the energy gained by an electron accelerated through one million volts. A snowflake striking the concrete pavement releases about 4 MeV.
fast neutron: a free neutron with a kinetic energy of at least 1 MeV (traveling faster than 14,000 km/sec). Nuclear reactions (fission and fusion) produce fast neutrons.
thermalize : to slow the effective speed of a subatomic particle (usually a neutron) until it corresponds to the speeds of like particles at the local temperature.
u Our oceans have a total of 1.43 × 1024 grams of water. For every 18 grams of water (1 mole) there are 6.022 × 1023 (Avogadro’s number) water molecules—each with 2 hydrogen atoms. One out of every 6,400 hydrogen atoms in our oceans is heavy hydrogen (2H, called deuterium). Each fast neutron thermalized by water produced at least 1 MeV of heat energy. (1 MeV = 1.602 × 10-6 erg) A hydrogen atom (1H) that absorbs a fast neutron releases 2.225 MeV of binding energy and becomes deuterium. So, assuming Earth had no unusual amount of deuterium before the flood, the amount of nuclear energy that was added to the subterranean water over several weeks, just in forming deuterium, was:
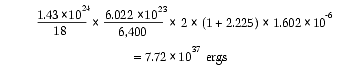
This is the energy that would be released by 1,800 trillion 1-megaton hydrogen bombs ! [See Endnote 3 on page 342.] The crust became an Earth-size nuclear engine during the several weeks this nuclear energy was being generated. This is a conservative estimate of the nuclear energy added to the subterranean water, because other products of nuclear fission and decay would have added additional energy, and some water was expelled permanently from Earth. Energy was also required to form radioisotopes and, in effect, “lift” them high above the floor of the valley of stability; energy was also absorbed in forming some elements heavier than iron.
The above calculation shows why so much deuterium was in the subterranean chamber. The solar system and stars contain little deuterium (a fragile isotope), but comets and asteroids contain large amounts of deuterium. (The comet chapter, pages 301–336, explains why the water in comets came from the subterranean chamber.)
This huge energy release (7.72 × 1037 ergs) must first be seen from the perspectives of two calculations: (a) and (b) below. From the first, this energy will appear small, but from the second, it will seem too large. Then, to help resolve both, consider the remarkable ability of water—especially supercritical water—to absorb and transfer heat and expel that energy into outer space as kinetic energy in the fountains of the great deep. Some of that energy is still being expelled from what was the porous floor of the subterranean chamber. [See Figure 56 on page 125.]
a . If 7.72 × 1037 ergs of energy were released uniformly in the Earth’s crust over 40 days, how many watts of power would be emitted in every cubic centimeter?
Earth has a surface area of 5.1 × 1018 cm2. Assuming the crust is 97 × 105 cm thick (about 60 miles), the average cubic centimeter of rock would generate only 0.05 watts.
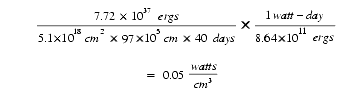
where a watt-day = 8.64 × 1011 ergs. A 100-watt light bulb releases energy almost 2,000 times faster. (Some 20-watt light bulbs are less than a cubic centimeter.)
b . If 7.72 × 1037 ergs of thermal energy were evenly distributed throughout the Earth at one time, the Earth would melt ! Earth’s mass is 5.976 × 10 27 grams. Let’s assume that a rise in Earth’s temperature of 1,784 K throughout would melt the Earth. Using the outer core’s specific heat and heat of fusion given in Table 109 on page 344, and neglecting the variation of these properties with pressure and temperature, the energy needed to melt the entire Earth is
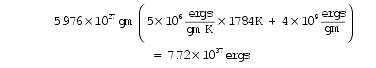
90. No liquid, including water, boils at its “boiling point.” The erroneous term arose before the mechanism of boiling was understood. To boil, a liquid’s temperature must be somewhat above its so-called boiling point.
I once demonstrated this to friends in our heat-transfer laboratory at MIT, by showing how hard it was to boil from a perfectly smooth metal surface, one that had no surface cracks or valleys—liquid mercury. I placed liquid mercury in the bottom of a very clean beaker and then poured pure water (doubly distilled and degassed) on top. As the beaker was heated by radiation lamps, the water’s temperature rose to 247°F (35 degrees above water’s “boiling point” at atmospheric pressure). Clouds of steam increasingly rolled out of the beaker, but no boiling occurred. Then, a very large bubble suddenly grew from a nucleation site (a little pit) in a tiny, barely visible dust particle in my “clean” water. The bubble grew and rose so fast that the water splashed off the ceiling. The highly agitated water molecules in the liquid (with 35 degrees of superheat) were frantically seeking a vapor pocket into which they could jump. Probably there were millions of sub-microscopic vapor pockets, but their effective radius was so small that the surrounding liquid’s surface tension was so powerful that the pressure inside was too high to form water vapor (steam).
A liquid’s so-called boiling point is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid.
91. Yes, as the temperature of the SCW slowly increases, the average radius (r) of the microscopic liquid droplets becomes even smaller, so the surface tension (the inter-molecular forces) squeezing the droplets increases as 1/r. Therefore, the pressure within the liquid droplets becomes much greater than the surrounding vapor’s pressure. Simultaneously, as the average liquid droplet becomes smaller through evaporation, the vapor’s density increases, so more vapor molecules merge at a faster rate to become microscopic liquid droplets, and more water molecules are ionized.
92. See “Oil—and Mountains of Salt—All in the Right Places” on page 396.
93. Granite typically has a tensile strength of 1,850 psi and a modulus of elasticity of 7,300,000 psi. Earth’s crust has a mean circumference of 24,875 miles. Therefore, the strain just before the rupture was about
Although other factors were involved, this might be, within an order of magnitude, the initial width of the rupture.
94. See "Frequency of the Fluttering Crust" on page 347.
95. See page 471 for confirmation of this in the paragraph quoted from The Book of the Cave of Treasures (A.D. 300–599).
96. Even dry salt (the mineral halite, NaCl) has a density 2.2 times than of water, while sediments have a density of about 3 times that of water.
97. “Structural features such as salt domes and anticlines [an archlike upfold] are perhaps the most likely place to find hydrocarbons.” Allen L. Hammond, “Bright Spot: Better Seismological Indicators of Gas and Oil,” Science, Vol. 185, 9 August 1974, pp. 515–517.
u “Sand and sandstone formations make excellent reservoirs [for oil], and carbonate rocks [such as salt domes] may also be good.” Arthur N. Strahler, Physical Geology (New York: Harper & Row, Publishers, 1981), p. 566.
98. The subterranean water reaches extremely high horizontal velocities as the chamber’s ceiling is pressed closer and closer to the chamber’s floor. See Figure 76 on page 145 and notice what happens as y approaches zero.
99. A. A. Vostrikov et al., “The Effect of Thermal Explosion in a Supercritical Water,” Technical Physics Letters, Vol. 27, 2001,pp. 847–849.
This paper describes the burning of certain hydrocarbons in SCW in a narrow temperature and pressure range. Methane (CH4) and carbon dioxide (CO2) were produced. It seems most likely that under different temperature and pressures, a wide variety of hydrocarbons would be produced.
For example, SCW is seen jetting up from the floor of the Gulf of Mexico. That water is coming from the pore spaces in what remained of the preflood subterranean chamber floor. As that SCW enters the cold water immediately above the sea floor, asphalt (tar) is deposited.
u “Even Jules Verne didn’t foresee this. Down at the bottom of the Atlantic Ocean is the hottest water on Earth, in a ‘supercritical’ state never seen before in nature ... and could offer a glimpse of how minerals such as gold, copper and iron are leached out of the entrails of the Earth and released into the oceans. Its water, but not as we know it ... .” Catherine Brahic, “Superheated Water Spews from the Seabed,” New Scientist, Vol. 198, 9 August 2009, p. 14.
u “Some tubeworm aggregations were completely embedded in solidified tar, indicating that they were later overcome by flows.” I. R. MacDonald et al., “Asphalt Volcanism and Chemosynthetic Life in the Campeche Knolls, Gulf of Mexico,” Science, Vol. 304, 14 May 2004, p. 1000.
u Martin Hovland et al., “Chapopote Asphalt Volcano May Have Been Generated by Supercritical Water,” Eos, Vol. 86, 18 October 2005, pp. 397–398.
100. In about 1982, I received a phone call from a scientist who, in 1942, participated at one of the most significant and dangerous experiments of all time. Enrico Fermi and his team had built the first nuclear reactor under the south side of the University of Chicago’s football stadium. It was a key step in the development of the atomic bomb.
One of the fascinating details he shared was that they could measure with a Geiger counter the radiation building up in the room (a squash court), and knew that neutrons were buzzing all around and through their bodies. He also said that the one thing they knew about atoms was that their nuclei were continually vibrating.
101. George F. Bertsch, “Vibrations of the Atomic Nucleus,” Scientific American, Vol. 248, May 1983, p. 64.
102. Imagine that you are pushing a child in a swing. The swing has a natural frequency, perhaps one cycle every two seconds. If you push the child ten times per second or once every ten seconds, you won’t get good results. It is best to push at the natural vibrational frequency of the swing (once every two seconds). But that is not enough. If each of your pushes at the resonant frequency puts more energy (a force moving through a distance) into the pendulum-like swing than is lost by various types of friction, the swing’s amplitude steadily increases.
The same thing happens in a nucleus whose vibrations are driven by streams of bremsstrahlung radiation, originating during the compression event from trillions of locations in the suddenly compressed hydroplates. Each stream contains some of the resonant frequencies—about 5 × 1021 cycles (or “pushes”) per second. Amplitudes steadily increase and nuclei are repeatedly distorted into unstable shapes and unstable internal configurations. Accelerated decay follows.
103. George Gamow, “Expanding Universe and the Origin of Elements,” Physical Review, Vol. 70, October 1946, pp. 572–573.
104. “However, it was soon realized that the building up of heavy nuclei during the Big Bang could not have continued very far, because collisions between nuclei became less frequent as the universe cooled [and expanded], and the thermal energy of the nuclei became too low to overcome the electrostatic repulsion of their positive charges.” Edward M. Baum et al., Nuclides and Isotopes: Chart of the Nuclides, 16th edition (Schenectady, NY: Knolls Atomic Power Laboratory, 2002), p. 34.
105. “Elevated emanations of hydrogen, radon, helium, and other gases were detected over some of the lineaments, thus indicating anomalous permeability of these zones in comparison with adjacent areas.” O. V. Anisimova and N. V. Koronovsky, “Lineaments in the Central Part of the Moscow Syneclise and Their Relations to Faults in the Basement,” Geotectonics, Vol. 41, No. 4, 2007, p. 315.
106. “... many lineaments are zones of seismic activity ... .” Ibid.
u “... the main seismic activity is concentrated on the first and second rank lineaments, and some of [the] important epicenters are located near the lineament intersections. Stich et al., (2001) obtained from the analysis of 721 earthquakes with magnitude between 1.5 and 5.0 mb [body-wave magnitude] that the epicenters draw [lie along] well-defined lineaments and show two dominant strike directions N120–130°E and N60–70°E, which are coincident with known fault systems in the area and with the source parameters of three of the largest events.” A. Arellano Baeza et al., “Changes in Geological Faults Associated with Earthquakes Detected by the Lineament Analysis of the Aster (TERRA) Satellite Data,” Pagina Web De Geofisica, December 2004, p. 1.
107. Ralph A. Alpher, Hans Bethe, and George Gamow, “The Origin of Chemical Elements,” Physical Review, Vol. 73, April 1948, pp. 803–804.
108. “As already mentioned, there is no stable nucleus with five or eight nuclear particles [nucleons], so it is not possible to build nuclei heavier than helium by adding neutrons or protons to helium ( 4He) nuclei, or by fusing pairs of helium nuclei. (This obstacle was first noted by Enrico Fermi and Anthony Tukevich.)” Steven Weinberg, The First Three Minutes (New York: Bantam Books, Inc., 1977), p. 119.
u The barrier at 5 nucleons causes almost instantaneous decays, with half-lives of less than 7.6 × 10-22 seconds.
109. “But the stellar theory of nucleosynthesis also had its problems. It is difficult to see how stars could build up anything like a 25–30 percent helium abundance—indeed, the energy that would be released in this fusion would be much greater than stars seem to emit over their whole lifetime.” Weinberg, p. 120.
110. “A third alpha particle therefore has to be captured nearly simultaneously with the collision of the original pair [of alpha particles] for 12C to be formed. This process is known as the triple-alpha reaction, and was first proposed in 1952. Oxygen is then created when 12C captures a fourth alpha particle.” Sofia Quaglioni, “Close Encounters of the Alpha Kind,” Nature, Vol. 528, 3 December 2015, p. 42.
111. Serdar Elhatisari et al., “Ab Initio Alpha—alpha scattering,” Nature, Vol. 528, 3 December 2015, p. 111–114.
112. “It seems probable that the elements all evolved from hydrogen, since the proton is stable while the neutron is not. Moreover, hydrogen is the most abundant element, and helium, which is the immediate product of hydrogen burning by the pp chain and the CN cycle, is the next most abundant element.” Burbidge et al., p. 549.
113. Joseph Silk, The Big Bang (San Francisco: W. H. Freeman and Co., 1980), p. 79.
114. See Endnote 33 on page 140.
115. Charles Seife, “Accelerator Aims to Find the Source of All Elements,” Science, Vol. 298, 22 November 2002, p. 1544.
u Other evolutionist journals also admit this.
Stars cook up nearly all of the approximately 60 atomic elements in people’s bodies. But exactly how that works remains a mystery. Dolly Setton, “The Cosmic Recipe for Earthlings,” Discover, September 2013, p. 10.
116. “... the temperatures in the interior of stars are measured in tens of millions of degrees, whereas several billion degrees are needed to ‘cook’ radioactive nuclei from the nuclei of lighter elements.” George Gamow, One Two Three ... Infinity, Bantam Science and Mathematics edition (New York: The Viking Press, Inc., 1961), p. 329.
Notice that researchers at the Proton-21 Electrodynamics Research Laboratory in the Ukraine, using a Z-pinch, are overcoming Coulomb forces and producing heavy elements by fusion at close to these billion-degree temperatures. [See page 384.] However, it happens briefly (in 10-8 second) in a “hot dot” that is less than 10-7 millimeter in diameter. Supernovas are not needed, only a focused and concentrated plasma.
117. If supernovas produced all the chemical elements that are heavier than iron (and their isotopes), supernova debris should show spectroscopically all those elements produced by the r-process (rapid process) for the capture of neutrons. It should be a simple matter to show thousands of heavy isotopes present in the spectrographs of supernova remnants.
...we have no spectroscopic evidence that r-process elements have truly been produced. Stephen Rosswog, “Radioactive Glow as a Smoking Gun,” Nature, Vol. 500, 29 August 2013, p. 536.
Cobalt-56 and cobalt-57 are seen in supernova remnants, causing some to claim that cobalt is produced by supernovas. The current theoretical understanding of the events leading to a supernova have nickel decaying into cobalt before the supernova, thereby powering the supernovae. The cobalt was not produced by the supernova.
The nickel decays radioactively into cobalt, which then decays radioactively into iron, powering the supernova’s incandescence. Yudhijit Bhattacharjee, “Death of a Star,” Science, Vol. 339, 4 January 2013, p. 23.
118. “Models indicate that supernovae do not create enough of the elements heavier than iron to account for the amounts of these elements found in the universe.” Neil F. Comins and William J. Kaufmann, Discovering the Universe (New York: W. H. Freeman and Co., 2009), p. 238.
119. “The simplest interpretation of this linear relation is that the radioactivity measured at the surface is constant from the surface to depth b.” Roy et al., p. 1.
Roy then calculates that throughout the eastern United States, b = 4.68 miles, but increases slightly for other regions, such as the western United States and parts of Australia.
120. If the base of a semi-infinite, 4.68-mile-thick slab of rock is heated from below by a steady heat source, half that heat flux will pass through the top of the slab in 1.5-million years. After 40-million years, 90% of the heat flux entering from below would reach the surface. For each doubling of the slab’s thickness, the time required for a given fraction of the heat flux to reach the surface increases by a factor of four.
121. Arthur H. Lachenbruch, “Crustal Temperature and Heat Production: Implications of the Linear Heat-Flow Relation,” Journal of Geophysical Research, Vol. 75, No. 17, 10 June 1970, pp. 3291–3300.
122. “Heat production rate is well correlated to lithology; no significant variation with depth, neither strictly linear nor exponential, is observed over the entire depths of the [two German holes].” Christoph Clauser et al., “The Thermal Regime of the Crystalline Continental Crust: Implications from the KTB,” Journal of Geophysical Research, Vol. 102, No. B8, 10 August 1997, p. 18,418.
123. Frank D. Stacey, Physics of the Earth (New York: John Wiley & Sons, 1969), p. 244.
124. Frank D. Stacey, Physics of the Earth, 3rd edition (Brisbane, Australia: Brookfield Press, 1992), pp. 62–65.
125. “Even larger amounts of neutrons can be generated [by bremsstrahlung radiation in heavy chemical elements], in particular in natural uranium.” Shkolnikov and Kaplan, p. 165.
126. Josh Dean, “This Machine Might Save the World,” Popular Science, January 2009, pp. 64–71.
127. “ [At the Oklo reactor] most of the fission-product elements and the neutron capture products have remained partially or wholly in place.” George A. Cowan et al., “The Oklo Phenomenon,” p. 342.
128. “Helium-3 occurs as a primordial nuclide, escaping from the Earth’s crust into the atmosphere and into outer space over millions of years.” http://en.wikipedia.org/wiki/Helium-3.
129. Frank D. Stacey, Physics of the Earth (New York: John Wiley & Sons, 1969), p. 240.
130. Dehydroxylation is the removal of hydroxide ions (OH-) from a mineral’s crystalline structure by the application of heat and high pressures. Usually the heat and pressure are applied to a large mass of the mineral. However, in the case at hand, a 218Po atom impacting a mineral containing hydroxide would concentrate tremendous heat and pressure near the impact point, release thousands of OH- ions from their crystalline structure, form water (HOH), and result in dehydroxylation. The reaction is of the type
[See Douglas Yeskis et al., “The Dehydroxylation of Kaolinite,” American Mineralogist, Vol. 70, 1985, pp. 159–164.] Flowing water then dissolves and removes the O 2– ion.
To appreciate the large number of particles that might be removed by the impact of just one 218Po atom—or the decay of an embedded 218Po atom—consider the following. At 100°C and atmospheric pressure, 539 calories of heat will evaporate 1 gram of liquid water. (1 MeV = 3.83 × 10 -14 cal) Eighteen grams of water (1 mole) contains 6.022 × 10 23 molecules. Therefore, the kinetic energy of one recoiling 218Po (2% of the 5.49 MeV of energy released by the decay of 222Rn) could, if concentrated, evaporate up to
131. After etching mica sheets with acid, Robert Gentry could see tiny pits where heavy, recoiling atoms had impacted after ejecting an alpha particle. He assumed those pits were made by recoiling polonium. Pit densities near isolated polonium halos were no greater than the pit densities far from halos. Therefore, he concluded that diffusion or slow movement did not transport polonium (an alpha emitter) into the halo centers. If that had happened, some polonium would have decayed as the polonium converged on those centers, so pit densities would have been greater near polonium halos. [See Robert V. Gentry, “Fossil Alpha-Recoil Analysis of Certain Variant Radioactive Halos,” Science, Vol. 160, 14 June 1968, pp. 1228–1230.] This led to his eventual conclusion that the hundreds of millions of polonium isotopes must have been clustered at specific points since the instant of creation.
However, Gentry overlooked the powerful positive electrical charges at certain impact points and the rapid transport of 222Rn in flowing water along channels between growing sheets of mica. [See "Frequency of the Fluttering Crust" on page 347.] A flowing 222Rn atom that emitted an alpha particle instantly became 218Po with a -2 electrical charge. That new polonium was pulled into the nearest point of positive charge in seconds. Then, when the anchored polonium decayed minutes later, heat from its recoil evaporated more negatively charged hydroxide particles, so those points became even more positively charged and attracted more polonium even faster from greater distances. Almost all the uniformly distributed recoil pits Gentry saw were produced by decaying 222Rn, not decaying polonium.
132. Ejaz ur Rehman et al., “Mass Spectrometric Determination of 234U/238U Ratio with Improved Precision,” Analytical Chemistry, Vol. 77, 1 November 2005, pp. 7098–7099.
133. This short-sighted argument was raised by Stanley Freske, in “Evidence Supporting a Great Age for the Universe,” Creation/Evolution,” Fall 1980, p. 38.
134. Richard A. Kerr, “Meteorite Mystery Edges Closer to An Answer—Or the End of a Field,” Science, Vol. 341, 12 July 2013, p. 126.
135. This is a major problem for evolutionists who visualize chondrules being formed at the extremely low pressures and temperatures of outer space. (At low pressures, volatiles bubble out quickly—like gas escaping from the sudden opening of a carbonated beverage.) However, the hydroplate theory explains the retention of volatiles, because they formed under the high confining pressures inside rocks in the subterranean chamber. Also, they froze seconds after escaping from the hot, high-pressure, subterranean chamber. [See “Rocket Science” on pages 322–323.]
136. Naoyuki Fujii and Masamichi Miyamoto, “Constraints on the Heating and Cooling Processes of Chondrule Formation,” Chondrules and Their Origins, editor Elbert A. King (Houston: Lunar and Planetary Institute, 1983), pp. 53–60.
u Impact melting would not duplicate characteristics in and around chondrules. [See J. A. Wood and H. Y. McSween Jr., “Chondrules as Condensation Products,” Comets, Asteroids, Meteorites, editor A. H. Delsemme (Toledo, Ohio: The University of Toledo, 1977), pp. 365–373. Also see T. J. Wdowiak, “Experimental Investigation of Electrical Discharge Formation of Chondrules,” Chondrules and Their Origins, pp. 279–283.] Donald E. Brownlee et al. give seven other reasons why impact melting did not produce chondrules. [See “Meteor Ablation Spherules as Chondrule Analogs,” Chondrules and Their Origins, p. 23.]
137. T. D. Swindle et al., “Radiometric Ages of Chondrules,” Chondrules and Their Origins, pp. 246–261.
u “CAIs [calcium-aluminum-rich inclusions] are believed to have formed about two million years before the chondrules. Here we report the discovery of a chondrule fragment embedded in a CAI.” Shoichi Itoh and Hisayashi Yurimoto, “Contemporaneous Formation of Chondrules and Refractory Inclusions in the Early Solar System,” Nature, Vol. 423, 12 June 2003, p. 728. [See also “Mixed-Up Meteorites” on page ix and “A Question of Timing” on page 691.]
138. Richard Ash, “Small Spheres of Influence,” Nature, Vol. 372, 17 November 1994, p. 219.
139. “As already described, the separated chondrules in the polished mount frequently grade into material similar to the matrix around their peripheries. ... boundaries between chondrules and matrix are frequently very gradational.” R. M. Housley and E. H. Cirlin, “On the Alteration of Allende Chondrules and the Formation of Matrix,” Chondrules and Their Origins, p. 152.
140. These researchers include: A.G. W. Cameron, E. Levy, S. Love, J. Wasson, and Fred L. Whipple. Whipple specifically refers to the Z-pinch as necessary to focus enough energy to suddenly melt tiny chondrules. [See Fred L. Whipple, “Chondrules: Suggestion Concerning the Origin,” Science, Vol. 153, 1 July 1966, pp. 54–56.]
141. Alan E. Rubin, “Secrets of Primitive Meteorites,” Scientific American, Vol. 308, February 2013, p. 41.
142. “Clear evidence of [former] 60Fe in chondrites was first found in troilite (FeS) and magnetite (Fe3O4).” Shogo Tachibana et al., “60Fe in Chondrites: Debris from a Nearby Supernova in the Early Solar System?” The Astrophysical Journal, Vol. 639, 10 March 2006, pp. L87–L90.
u “ [Researchers] analyzed two primitive meteorites that are thought to be almost pristine leftovers of solar system formation. They detected nickel 60, the product of the radioactive decay of iron 60, in chemical compounds where, by rights iron should be found.” Simon F. Portegies Zwart, “The Long-Lost Siblings of the Sun,” Scientific American, Vol. 301, November 2009, p. 42.
u “Recent studies of meteorites confirm the presence of live 60Fe in the early solar system.” J. Jeff Hester et al., “The Cradle of the Solar System,” Science, Vol. 304, 21 May 2004, p. 1116.
143. What is meant by “quickly”? Supernovas are the hottest and most violent explosions observed in the universe. If mineral grains are somehow to form from a supernova, the gas/plasma debris from the supernova must first merge into microscopic particles. That is quite a trick, because the expanding gas/plasma moves radially outward, steadily increasing the distances between most of its atomic and subatomic particles. Martin Harwit calculates that to grow a grain to only 10-5 centimeter would require 3-billion years—assuming no expansion and that every particle that strikes a growing grain would stick. Sir Fred Hoyle put it more bluntly; “... there is no reasonable astronomical scenario in which mineral grains can condense.” [See “Interstellar Gas” on page 95.]
Second, these tiny grains (drifting weightlessly in space) must gravitationally collect into small bodies. Then, those bodies must somehow merge into asteroid-size bodies, massive enough to compress and heat (in a nearly absolute zero, environment) the grains into uniform crystals. At that point, enough 60Fe atoms might be concentrated to form minerals, such as troilite (FeS) and magnetite (Fe3O4). How long would this second step take? No one can say for sure, but probably most astronomers have an opinion. If they were candid, I suspect many would say that this second step couldn’t happen in 10,000,000 years. But almost all the 60Fe (half-life 1,500,000 years) would have decayed before then. Neither the first nor the second step could happen quickly enough to form detectable crystals containing 60Fe.
144. “The supernova was stunningly close; much closer to the sun than any star is today.” Brian D. Fields, as quoted by the University of Illinois News Bureau, 10 April 2006. See
http://news.illinois.edu/NEWS/06/1004solar.html
u Leslie W. Looney, John J. Tobin, and Brian D. Fields, “Radioactive Probes of the Supernova-Contaminated Solar Nebula,” The Astrophysical Journal, Vol. 652, 1 December 2006, pp. 1755–1762.
145. George Cooper et al., “Carbonaceous Meteorites As a Source of Sugar-Related Organic Compounds for the Early Earth,” Nature, Vol. 414, 20/27 December 2001, pp. 879–883.
146. Peter R. Briere and Kathryn M. Scanlon, “Lineaments and Lithology Derived from a Side-Looking Airborne Radar Image of Puerto Rico,” U.S. Geological Survey Open-File Report 00-006, 2000, pp. 1–5.
147. John W. Harbaugh et al., “Reconstructing Late Cenozoic Stream Gradients from High-Level Chert Gravels in Central Eastern Kansas,” Current Research in Earth Sciences, Bulletin 253, 2007, p. 14.
148. “The observation that Mars’ northern polar cap barely deforms [from season to season] implies that its planetary interior is colder than expected.” Matthias Grott, “Is Mars Geodynamically Dead?” Science, Vol. 320, 30 May 2008, p. 1171.
“This result is surprising. First, the temperatures in the interior of terrestrial planets should be proportional to their radius if they started with the same amount and distribution of radioactive, heat-producing elements and then cooled through surface losses. In this case, [the surface heat loss from] Mars would be expected to plot between Earth and the Moon. However, the new estimates imply that the martian heat flow, a measure for the temperatures in the planetary interior, is below that of the Moon, even though Mars is about twice the diameter.” Ibid.
u “Mars probably has subchondritic heat sources” [that is, less heat-generating radioactive material than is contained in the meteoritic material from which it supposedly formed]. Roger J. Phillips et al., “Mars North Polar Deposits: Stratigraphy, Age, and Geodynamical Response,” Science, Vol. 320, 30 May 2008, p. 118585.
149. Paul M. Myrow et al., “Extraordinary Transport and Mixing of Sediment across Himalayan Central Gondwana during the Cambrian-Ordovician,” Geological Society of America Bulletin, Vol. 122, September/October 2010, p. 1660.
150. Ping Wang et al., “Tectonic Control of Yarlung Tsangpo Gorge Revealed by a Buried Canyon in Southern Tibet,” Science, Vol. 346, 21 November 2014, p. 979.
u “The constant river gradient strongly suggests a rapid uplift event created the gorge, rather than the river incision as previously believed.” Stella Hurtley, “Tibetan Gorge Avoids a Tectonic Aneurysm,” Science, Vol. 346, 21 November 2014, p. 960.
151. Burbidge et al., pp. 547–650.
152. “Optical measurements of the beryllium and boron abundances in halo stars have been achieved by the 10 meter KECK telescope and the Hubble Space Telescope. These observations indicate a quasi linear correlation between Be and B vs. Fe, at least at low metallicity, which, at first sight, is contrary to a dominating GCR [Galactic Cosmic Ray] origin of the light elements which predicts a quadratic relationship. As a consequence, the theory of the origin and evolution of LiBeB nuclei has to be refined.” E. Vangioni-Flam and M. Cassé, p. 77.
153. “The Rn-222 alpha particle map shows that radon gas was emanating from the vicinity of craters Aristarchus and Kepler at the time of Lunar Prospector.” Stefanie L. Lawson et al., “Recent Outgassing from the Lunar Surface: The Lunar Prospector Alpha Particle Spectrometer,” Journal of Geophysical Research: Planets, Vol. 110, September 2005. p. E09009.
154. A blind test requires that the people making the measurements not know (be “blind” to) which of several specimens is the one of interest. For example, to measure a rock’s age by some radiometric technique, similar rocks—of different, but known, ages—must accompany the rock of interest. Only after the measurements are announced are the technicians making the measurements told the history of any specimen. Subtle biases can influence the experimental procedure if individuals with vested interests in the test’s outcome make the measurement or influence those who do. Blind tests ensure objectivity.
A special type of blind test commonly used in medicine is a “double-blind test.” Neither doctors nor patients know who receives the special treatment being tested. A random selection determines which patients receive the special treatment and which receive a placebo—something obviously ineffective, such as a sugar pill. Experienced medical researchers give little credibility to any medicine or treatment that has not demonstrated its effectiveness in a well-designed and rigorously executed double-blind test.
The Shroud of Turin, claimed to be the burial cloth of Christ, was supposedly dated by a blind test. Actually, the technicians at all three laboratories making the measurements could tell which specimen was from the Shroud. [Personal communication on 19 July 1989 with Dr. Austin Long, who participated in the radio-carbon dating.] The test would have been blind if the specimens had been reduced to unidentified carbon powder before they were given to the testing laboratories.
Actually, a more precise dating method for the Shroud had already been discovered. A Roman coin (a Pontius Pilate lepton) had been placed over the right eye of the man whose image was on the Shroud. That coin was minted between 29 AD and 32 AD. Discernible on the coin was a misspelled word, which further identifies the coin, because “at least four other Pilate coins currently exist that exhibit this misspelling.” Placing coins over the eyes of the deceased was a common burial practice in Jerusalem between the 1st Century BC through the 1st Century AD. [See Mark Antonacci, Test the Shroud at the Atomic and Molecular Levels (United States: LE Press, LLC, 2015), pp. 69-75.]
Radiometric dates that do not fit the favored theory are often thrown out by alleging contamination. Few ever hear about such tests. If those who object to a blind radiometric date have not identified the contamination before the test, their claims of contamination should carry little weight. Therefore, careful researchers should first objectively evaluate the possibility of contamination.
Humans are naturally biased. We tend to see what we want to see and explain away unwanted data. This applies especially to those proposing theories, myself included. Scientists are not immune to this human shortcoming. Many popular ideas within geology would probably never have survived had a critical age measurement been subjected to a blind test.
155. John Woodmorappe, “Radiometric Geochronology Reappraised,” Creation Research Society Quarterly, Vol. 16, September 1979, pp. 102–129.
u Robert H. Brown, “Graveyard Clocks: Do They Tell Real Time?” Signs of the Times, June 1982, pp. 8–9.
u “It is obvious that radiometric techniques may not be the absolute dating methods that they are claimed to be. Age estimates on a given geological stratum by different radiometric methods are often quite different (sometimes by hundreds of millions of years). There is no absolutely reliable long-term radiological ‘clock.’ ” William D. Stansfield, Science of Evolution (New York: Macmillan Publishing Co., 1977), p. 84.
156. “Chemical and physical processes such as mantle convection, tectonic-plate recycling and magma generation through partial melting should have scrambled, if not obliterated, any coherent geochemical signature of the primordial material. Even if a vestige of such material remained, it seems unlikely that it would be found in any samples from Earth’s surface or the shallow subsurface that are available to geologists. Yet that is what [this] new evidence suggests.” David Graham, “Relict Mantle from Earth’s Birth,” Nature, Vol. 466, 12 August 2010, p. 822.
u “Cenozoic-Era Baffin Island and West Greenland lavas, previously found to host the highest terrestrial-mantle 3He/ 4He ratios, exhibit primitive lead-isotope ratios that are consistent with an ancient mantle source age of 4.55–4.45 Gyr [billion years]. The Baffin Island and West Greenland lavas also exhibit 143Nd/ 144Nd ratios similar to values recently proposed for an early-formed (roughly 4.5 Gyr ago) terrestrial mantle reservoir.” Matthew G. Jackson et al., “Evidence for the Survival of the Oldest Terrestrial Mantle Reservoir,” Nature, Vol. 466, 12 August 2010, p. 853.
157. “Beyond its Fe deficiency, the singular feature of HE0107–5240 is that its measured abundance of C, relative to Fe, is about 10,000 times the observed ratio of these elements in the Sun, the largest such ‘over-abundance’ ratio ever seen. The N abundance ratio is also greatly enhanced, though only by a factor of 200.” Timothy C. Beers, “Telling the Tale of the First Stars,” Nature, Vol. 422, 24 April 2003, p. 825.
158. Beth Geiger, “Relics of Earth’s Birth Still Linger,” Science News, Vol. 189, 11 June, 2016, p. 13.
159. Hanika Rizo et al., “Preservation of Earth-Forming Events in the Tungsten Isotopic Composition of Modern Flood Basalts,” Science, Vol. 352, 13 May 2016, pp. 809–812.
160. “The ancient remnants somehow escaped being mixed by convection currents in the mantle.” Geiger, p. 13.
161. Silk, p. 124.
162. Baum et al., p. 34.