1. Scott A. Sandford et al., “Organics Captured from Comet 81P/Wild 2 by the Stardust Spacecraft,” Science, Vol. 314, 15 December 2006, pp. 1720–1724.
u Bill Steigerwald, “NASA Researchers Make First Discovery of Life’s Building Block’s in Comet,” NASA Goddard Space Flight Center, 17 August 2009,
www.nasa.gov/mission_pages/stardust/news/stardust_amino_acid.html
u “Other clues about [comet 67P’s] origins came from the spacecraft’s chemical sensors. Scanning the surface, for instance, a spectrometer detected an absorption feature associated with complex organic molecules that could include carboxylic acids—precursors to amino acids. ... NASA’s Stardust mission found actual amino acids in comet dust it sampled in 2004—but the molecules Rosetta has detected are more complex than those seen on other comets.” [A final list of these complex molecules is in Table 14 on page 311.] Eric Hand, “Comet Close-up Reveals a World of Surprises,” Science, Vol. 347, 23 January 2015, pp. 358–359.
u F. Capaccioni et al., “The Organic-Rich Surface of Comet 67P/Churyumov-Gerasimenko as Seen by VIRTIS/Rosetta,” Science, Vol. 347, 23 January 2015, pp. 389.
2. “We know that it is hard to find a comet without the spectral features of C2 , C3 , and CN in their comas. Huggins was struck by the fact that the material in the comets was similar to organic matter of unquestioned biological origin on Earth. Many scientists cautiously concluded that the carbon compounds found by [William] Huggins [in 1868] in the comas of comets were, as one of his contemporaries wrote, ‘the result of the decomposition of organic bodies.’ ” [emphasis in original] Carl Sagan and Ann Druyan, Comet (New York: Ballantine Books, 1997), p. 148.
u “Recent observations of comet celebrities Halley, Hale-Bopp and Hyakutake [Hyah-koo-tah-kay] revealed that these icy visitors are rife with organic compounds. In 1986 cameras on board the Giotto and Vega spacecrafts captured images of dark material on Halley’s surface that resembles the coallike kerogen in some meteorites, and mass spectrometers caught glimpses of carbon-rich molecules. More recently, ground-based telescopes inspecting the coma and tail of comets Hyakutake and Hale-Bopp distinguished a number of specific organic compounds, including methane and ethane.” Max P. Bernstein et al., “Life’s Far-Flung Raw Materials,” Scientific American, Vol. 281, July 1999, p. 45.
3. M. A. Cordiner et al., “Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) using the Atacama Large Millimeter/Submillimeter Array,” The Astrophysical Journal Letters, Vol. 792, 1 September 2014, pp. 1–6.
u “Astronomers have captured three-dimensional images of organic compounds streaming from two comets [in clumps: hydrogen cyanide (HCN), hydrogen iso-cyanide (HNC), and formaldehyde (H2CO)].” Martin Cordiner, “Comets Forge Organic Molecules,” Nature, Vol. 512, 21 August 2014, p. 234.
u “The COSAC [COmetary SAmpling and Composition apparatus] gas analyzing instrument on Philae [the Rosetta lander that landed on Comet 67P on 12 November 2014] was able to ‘sniff’ the atmosphere and detect the first organic molecules after landing, the DLR German Aerospace Center said.” Victoria Bryan, “Comet Team Detects Organic Molecules, Basis of Life on Earth,” www.reuters.com/article/ 2014/11/18/us-space-comet-idUSKCN0J21V520141118
The comet’s atmosphere contained methane, the simplest organic molecule. Methane almost always comes from life, which means that life (such as bacteria) once was, or is, probably on Comet 67P. In rare cases, methane can be produced in other ways, such as when liquid water interacts with certain rocks. However, comets are too cold to have liquid water. Even if comets heated up by traveling close to the Sun or by an impact, the comet’s ice would immediately become a gas (steam), never liquid water. Therefore, bacteria probably were or are on Comet 67P—bacteria launched from Earth. Don’t be fooled by claims that life on Earth came from comets or extraterrestrial bodies. Those ideas, called panspermia, beg the question of how life began, ignore all the deadly radiation in space, and don’t tell us what the critters ate. As shown in Table 14 on page 311, in 2009 scientists discovered many highly complex organic molecules such as glycine, on a comet. Glycine definitely came from life.
4. If A and B have a similar and unusual characteristic, or they correlate, some might claim that A caused B. But maybe B caused A—or C caused A and B. Perhaps no cause-and-effect link exists. Many humorous stories, scams, and even misguided scientific efforts are rooted in this logical fallacy—seeing a relationship and, with no other information, assuming a specific cause produced an effect.
Because (A) traces of organic molecules are found in comets, and (B) organic molecules are found in every living thing on Earth, did comets bring life to Earth (A caused B)? Maybe comets and organic molecules came from Earth (B caused A). We should consider all possibilities. Many who leap to conclude that comets explain life on Earth know how difficult it is to explain life originating by natural processes. Most authorities will privately admit that life is so complex that they can’t imagine how it could form anywhere. [See pages 13–25.] Desperation may force this poor logic—that comets brought life to Earth. But even if comets did, how did comets acquire life? It takes more than time and distance.
Be aware that organic molecules—which are simply molecules containing hydrogen plus carbon rings or chains—are as far from becoming life as bricks are from becoming the Empire State Building. Yes, bricks might form naturally in a dried-up stream bed, but I cannot imagine the Empire State Building forming by natural processes. If you saw a large pile of bricks mixed with steel, tubes, wire, glass, and insulation, would you conclude that a building was evolving or had been destroyed? Great intelligence is needed to produce life.
5. The Deep Impact space mission found that the nucleus of comet Tempel 1 had a density of 0.62 gm/cm3 and was about 60% empty space. If the dirt’s density was 2.7 gm/cm3 and the ice’s density was 0.92 gm/cm3, it can be shown that about 38% of the comet, by mass, was water. [See M. F. A’Hearn et al., “Deep Impact: Excavating Comet Tempel 1,” Science, Vol. 310, 14 October 2005, p. 262, and Richard A. Kerr, “Deep Impact Finds a Flying Snowbank of a Comet,” Science, Vol. 309, 9 September 2005, p. 1667.]
6. G. Gloeckler et al., “Interception of Comet Hyakutake’s Ion Tail at a Distance of 500 Million Kilometers,” Nature, Vol. 404, 6 April 2000, pp. 576–578.
7. John Fleck, “Comets Showered Ice on Moon,” ABQ Journal of Science & Technology, 3 September 1998, p. C3.
8. “Infrared spectroscopic measurements of the lunar surface from [three] spacecraft provide unambiguous evidence for the presence of hydroxyl (OH) or water [or both].” Paul G. Lucey, “A Lunar Waterworld,” Science, Vol. 326, 23 October 2009, p. 531.
9. W. C. Feldman et al., “Fluxes of Fast and Epithermal Neutrons from Lunar Prospector: Evidence for Water Ice at the Lunar Poles,” Science, Vol. 281, 4 September 1998, p. 1496.
10. Nancy L. Chabot et. al., “Images of Surface Volatiles in Mercury’s Polar Craters Acquired by the MESSENGER Spacecraft,” Geology, Vol. 42, December 2014, pp. 1051–1054.
11. “But the association of comets with catastrophe remains curiously steady through the generations.” Sagan and Druyan, p. 279.
u “Here, as indeed among all peoples generally, comets are regarded as omens of disaster.” Fred Hoyle and Chandra Wickramasinghe, Lifecloud (New York: Harper & Row, Publishers, 1978), p. 99.
12. Nigel Calder, The Comet Is Coming! (New York: The Viking Press, 1980), pp. 13, 26.
u Loren Coleman list several “suicide epidemics” in his book, The Copycat Effect (Simon & Schuster, Inc., New York, 2004), pp. 77–79.
13. Isaac Newton, “Of the Attractive Forces of Spherical Bodies,” Proposition LXX, Theorem XXX, Section XII, Book I, The Principia (1687; reprint, Amherst, New York: Prometheus Books, 1995), p. 154. The shell must have uniform thickness and density.
14. The Apollo 13 astronauts had to abort their planned lunar landing, because an oxygen tank exploded soon after liftoff. This forced them to loop around the Moon and execute a tricky reentry back to Earth. Ground controllers had difficulty tracking the spacecraft by radar, because a cloud of urine orbited and partially hid the spacecraft. The astronauts were then told to hold all waste liquid in onboard containers. Today, astronauts avoid this problem by dumping waste material overboard just before igniting their rocket thrusters. Gravity, even that of a spacecraft, a rock, or a water droplet, acts on everything.
15. It can be shown that the radius of a sphere of influence (SoI) of a spherical rock of radius r that is moving away from Earth is about
where R is the Earth’s radius and h is the rock’s height above the Earth.
When many particles (rocks, dirt, ice, and water molecules, all moving away from Earth) interact and exchange momentum, their velocities become more similar. The effective SoI of the combined mass increases, so those particles will increasingly tend to merge.
16. An extremely rare exception might occur if one body strikes the other with a very delicate glancing blow. Another exception would be if a third particle passing by had just the right mass, speed, direction, and position so that its gravitational attraction could slow the droplet enough to cause capture. However, impacts and interfering third bodies are much more apt to cause scattering than capture.
17. Every body, even a dust particle or a star, has an escape velocity—that is, the slowest speed an arbitrary object needs from a specified point to escape that body’s gravity pull and go to infinity. The escape velocity for an object at the surface of the Earth, is 11.2 km/sec (7.0 mi/sec). For something at the surface of the Sun to escape the solar system, it is 617.2 km/sec (383.5 mi/sec). For something 1 AU from the Sun to escape the solar system requires 42.3 km/sec (26.3 mi/sec).
18. “Capture” is the proper term. Those who say stars, planets, and moons formed through capture often use the misleading terms “accrete,” “condense,” and “gravitational collapse,” which imply a “pulling in.” These words, while sounding scientific to a layman, reveal a misunderstanding of the physics. While gravity would move two isolated particles in space toward each other if their relative velocity were initially zero, particles in space are not isolated and seldom travel with the same speed and direction. For a body to capture a particle, (a) the particle must be within the body’s sphere of influence, (b) the particle’s velocity relative to the body must never carry it outside the sphere of influence, and (c) the body’s gravitational grip on the particle must increase, so later perturbations do not strip the orbiting particle away. Requirement (c) is most easily satisfied if the body has an atmosphere—a surrounding gas. [See "Evolving Planets?" on page 29.]
19. “It turns out to be surprisingly difficult for planetesimals to accrete mass during even the most gentle collisions.” Erik Asphaug, “The Small Planets,” Scientific American, Vol. 282, May 2000, p. 54.
u In 1805, Laplace first explained the “sphere of influence” concept, or, as he called it, the “sphere of attraction.” He applied it to planets acting on comets, but did not use it to show why permanent capture to form larger bodies, such as comets or planets, is so difficult. [See Nathaniel Bowditch, Celestial Mechanics by the Marquis de Laplace, Vol. 4 (Bronx, New York: Chelsea Publishing Co., 1966), pp. 417–437.]
20. Unfortunately, “short-period comets” have been arbitrarily defined as comets with periods less than 200 years. A more physically meaningful definition, used here, will be comets with periods less than 100 years, because there is a huge, recognized excess of such comets. Any acceptable theory of comet origins should explain this excess.
21. All orbital information was taken on 30 October 2016 from the Jet Propulsion Laboratory’s Small-Body Data Base at
http://ssd.jpl.nasa.gov/sbdb_query.cgi
22. Disregarding the effects of wind resistance, fired bullets and thrown balls are very briefly in elliptical orbits about Earth’s center of mass. Once they strike the Earth’s surface, their orbits end.
23. “Jupiter’s huge attractive mass has somehow collected two-thirds of all the short-period comets into a family. Saturn probably also plays a supporting role in the process. Jupiter and Saturn appear to be much more important in the story of comets than was indicated by their slight disturbances of the motion of Halley’s comet. The existence of Jupiter’s comet family is one of our important clues to the origin of comets.” Fred L. Whipple, The Mystery of Comets (Washington, D.C.: Smithsonian Institution Press, 1985), p. 74.
“What is the chance that Jupiter could catch them [comets falling from an Oort cloud] by its gravity and tame them into short-period, prograde orbits? He [H. A. Newton] found that the chance is very small. Only about one in a million would have its period reduced to less than Jupiter’s period of 11.86 years.” Ibid., p. 75.
24. “By comparing the orbital element distribution of JFCs [Jupiter’s family of comets] to that produced by our simulations we deduce that JFCs are statistically most likely to have physical lifetimes of about 12,000 years.” Harold F. Levison and Martin J. Duncan, “From the Kuiper Belt to Jupiter-Family Comets,” Icarus, Vol. 127, May 1997, p. 13.
u “But once so deflected [into short-period orbits], these comets must have comparatively short lifetimes, astronomically speaking, and probably no short-period comet can survive more than about 10,000 years.” R. A. Lyttleton, Mysteries of the Solar System (Oxford, England: Clarendon Press, 1968), p. 110.
25. “There is no example of a known short-period comet evolving into a long-period comet of small enough perihelion to be visible.” Edgar Everhart, “Examination of Several Ideas of Comet Origins,” The Astronomical Journal, Vol. 78, May 1973, p. 332.
26. Harold F. Levison and Martin J. Duncan, “The Long-Term Dynamical Behavior of Short-Period Comets,” Icarus, Vol. 108, March 1994, Figure 5, p. 25.
27. Many of these comets are so far away and have not been observed long enough for their orbital periods to be calculated.
28. This can be seen by recalling the “Hollow Shell” example on page 305. The Kuiper Belt (and to a lesser extent, the asteroid belt) can be thought of as approximately consisting of thousands of thin, concentric, spherical shells—like rings in an onion. A comet inside any shell would not feel its gravitational pull. However, comets outside a shell would feel, for up to thousands of years, a gravitational pull equal to that of the shell’s entire mass concentrated at the center of that shell.
29. “Many scientific papers are written each year about the Oort Cloud, its properties, its origin, its evolution. Yet there is not a shred of direct observational evidence for its existence.” Carl Sagan and Ann Druyan, p. 210.
However, Sagan and Druyan believed that the Oort cloud exists, and went on to predict (p. 211) that “with the refinement of our scientific instruments, and the development of space missions to go far beyond Pluto,” the cloud will be seen, measured, and studied. As of 2018, that prediction is unfulfilled.
30. Raymond A. Lyttleton, “The Non-Existence of the Oort Cometary Shell,” Astrophysics and Space Science, Vol. 31, December 1974, pp. 385–401.
Assuming the Oort cloud exists helps preserve the belief in a multibillion-year age for the solar system.
u “Recently, Lyttleton (1974) confirmed our conclusion of 1954: the Oort’s hypothetical cloud of comets cannot exist.” S. K. Vsekhsvyatsky, “Comets and the Cosmogony of the Solar System,” Comets, Asteroids, Meteorites, editor A. H. Delsemme (Toledo, Ohio: The University of Toledo, 1977), p. 470.
Vsekhsvyatsky estimated (p. 470) that considerably more than 1020 gm/yr of cometary matter are lost from the solar system. Over the supposed age of the solar system (4.5-billion years), lost comet mass would “nearly correspond to the total present mass of the planets.” He believed this was unreasonable. I agree.
“... many people would be happier if there were more objective evidence for the reality of the Oort Cloud.” John Maddox, “Halley’s Comet Is Quite Young,” Nature, Vol. 339, 11 May 1989, p. 95.
PREDICTION 35: The Oort cloud will never be detected, because it does not exist.
31. “Using current standard models for the formation of comets, a significant number of [hyperbolic] comets should have been observed. This lack of detections of extrasolar comets is becoming an embarrassment to the theories of solar system and cometary formation and may drive the parameters of these models.” Thomas A. McGlynn and Robert D. Chapman, “On the Nondetection of Extrasolar Comets,” Astrophysical Journal, Vol. 346, 15 November 1989, p. L105.
u “No comet on a clearly interstellar trajectory has been observed passing through the planetary system.” Paul R. Weissman, “Dynamical History of the Oort Cloud,” Comets in the Post-Halley Era, Vol. 1, editors R. L. Newburn et al. (Boston: Kluwer Academic Publishers, 1991), p. 479.
u “[Evolutionary] models predict that the Milky Way Galaxy should be awash with such ‘rogue’ objects, because huge numbers of asteroids and comets are thought to be ejected from young planet-forming systems. However, surveys have found no objects that have been confirmed as interstellar.” Francesca Cesari et al., “Rogue Asteroids May Be Rare,” Nature, Vol. 544, 13 April 2017, p. 141.
u “No comet has ever been observed on a trajectory originating outside the gravitational influence of the Sun. And yet, sooner or later, such comets should be seen.” Sagan and Druyan, p. 350.

PREDICTION 36: No incoming comet will ever be seen on a hyperbolic orbit, because cometary material came from Earth as the flood began, not from outside the solar system.
32. “A flaw in our understanding of the orbital evolution of comets is that the number of short-period comets—those with orbital periods less than 200 years, such as comet Halley—is much greater than theory predicts. The discrepancy is enormous; the observed number is two orders of magnitude larger than expected.” Julia Heisler, “Orbital Evolution of Comets,” Nature, Vol. 324, 27 November 1986, p. 306.
33. This expected distribution of comets, first shown mathematically by van Woerkom in 1948, has frequently been verified by powerful computer simulations. [See A. J. J. van Woerkom, “On the Origin of Comets,” Bulletin of the Astronomical Institutes of the Netherlands, Vol. 10, 8 December 1948, pp. 445–472.]
u A few researchers once believed that second-pass comets were not visible, because they dimmed after losing volatile gases on their first pass. This early loss of volatiles happens, but the effect is not strong. Comets moving away from the Sun are not appreciably dimmer than when they were at the same distance but approaching the Sun. [See M. C. Festou, “The Derivation of OH Gas Production Rates from Visual Magnitudes of Comets,” Asteroids Comets Meteors II, editors C. I. Lagerkvist et al. (Uppsala, Sweden: Uppsala University Press, 1986), pp. 299–303.]
Wiegert simulated 125,495 artificial comets in orbits 10,000 – 50,000 AU from the Sun. For 5 billion simulated years, the giant planets and the galactic tide perturbed the comets. Even when simulating comets that rapidly fade in visibility, Wiegert found that neither fading nor many other effects could explain the lack of observed long-period comets that have completed more than one orbit. [See Paul Arnold Wiegert, The Evolution of Long-Period Comets (Ph.D. dissertation, University of Toronto, 1996).]
u Thomas D. Nicholson, “Comets, Studied for Many Years, Remain an Enigma to Scientists,” Natural History, Vol. 75, March 1966, pp. 44–46.
u Lyttleton, Mysteries, p. 110.
u Hannes Alfven and Gustaf Arrhenius, Evolution of the Solar System (Washington, D.C.: NASA, 1976), p. 234.
34. “Since planetary perturbations typically change 1/a [a quantity proportional to energy per unit mass] by several hundred units during one revolution about the Sun, we were forced to conclude, following Oort, that the great majority of these [near-parabolic comets] comets were making their first passage through the inner part of the solar system.” Brian G. Marsden et al., “New Osculating Orbits for 110 Comets and Analysis of Original Orbits for 200 Comets,” The Astronomical Journal, Vol. 83, January 1978, p. 64.
35. “Hence, if comets like Hale-Bopp brought in the Earth’s water, they would have brought in a factor of 40,000 times more argon than is presently in the atmosphere.” T. D. Swindle and D. A. Kring, “Implications of Noble Gas Budgets for the Origin of Water on Earth and Mars,” Eleventh Annual V. M. Goldschmidt Conference, Abstract No. 3785 (Houston: Lunar and Planetary Institute, 20–24 May 2001).
Argon is not commonly found in outer space, and it is a nobel gas, so it doesn’t react chemically with other matter. How then did comets collect argon?
Argon was probably produced by solar wind striking chlorine in the frozen saltwater that comprises much of a comet. (Protons constitute 95% of the solar wind.) Protons (p) bombarding chlorine (Cl) produce argon (Ar) and a gamma ray (g), a process called proton capture. For example:
p + 35Cl 36Ar + g
This reaction would have begun long before comets gravitationally assembled from the material in the fountains of the great deep. After comets assembled, primarily the outer shell of comets would have been increasingly enriched with argon. The argon content in comet Hale-Bopp was measured in the gases that vaporized from Hale-Bopp’s outer shell, so we cannot assume that the entire comet had such a high argon enrichment. Nevertheless, if comets supplied most of Earth’s water, we probably would see much more argon in our atmosphere than we have, although it would not be 40,000 times as much.

PREDICTION 37: A greater concentration of argon will be found in the outer portions of comets.
Sodium, which few would expect to find in outer space, was one of the first chemical elements identified in comets. [See Donald K. Yeomans, Comets (New York: John Wiley & Sons, Inc., 1991), p. 217.]
36. “Comet investigators found levels of ethane in Comet Hyakutake that are about 1,000 times greater than can be explained if the molecules were formed by normal physical processes within the gases of the primordial solar nebula, the birth cloud of the Solar System.” Douglas Isbell and Jim Sahli, “Chemical Measurements of Comet Hyakutake Suggest a New Class of Comets,” NASA Press Release 96–108, 31 May 1996.
37. “Scientists have been catching hints of methane in the Martian atmosphere for 15 years using Earth-based telescopes, Mars orbiters and NASA’s Curiosity rover. As evidence of the gas has accumulated, the debate over its origin has intensified. ‘Nearly 95% of all methane in the Earth’s atmosphere originated from current and past biology’.” Nisha Gaind, “Probe Tackles Mars Methane Mystery,” Nature, Vol. 556, 26 April, 2018, p. 419.
38. “But an old reservoir of methane [on Mars] is problematic, Mumma says, because it would be hard to explain how it could be steadily released over billions of years. That would suggest that if bacteria are indeed the source of the methane, the organisms are active now.” Ron Cowen, “Plumes of Martian Methane Hint at Possible Underground Microbial Life,” Science News, Vol. 175, 14 February 2009, p. 10.
u “... the destruction lifetime for CH4 [methane] is much shorter than the time scale (~350 years) estimated for photochemical destruction. Another process thus must dominate removal of atmospheric CH4 on Mars, and it must be more efficient than photochemistry by a factor > 100.” Michael J. Mumma et al., “Strong Release of Methane on Mars in Northern Summer 2003,” Science, Vol. 323, 20 February 2009, p. 1044.
u Alexandra Witze, “Clues Emerge to Martian Mystery,” Nature, Vol. 563, 1 November 2018, pp18–19.
39. “Krasnopolsky’s team calculates that comets striking Mars couldn’t deliver enough methane to replace what’s lost.” Ron Cowen, “Martian Methane: Carbon Compound Hints at Life,” Science News, Vol. 165, 10 April 2004, p. 228.
40. Christopher R. Webster, et al. “Background Levels of Methane in Mars’ Atmosphere Show Strong Seasonal Variations,” Science, Vol. 360, pp. 1093–1096, 8 June 2018.
u “But in late March, researchers analyzing data from the European Mars Express satellite reported the planet’s atmosphere contains traces of methane, [usually] a by-product of bacteria here on Earth. Could this be the long-awaited sign of Martian life?” Maia Weinstock, “Our Favorite Martians,” Discover, Vol. 25, August 2004, p. 16.
u “Living systems produce more than 90% of Earth’s atmospheric methane; the balance is of geochemical origin [if liquid water is present].” Mumma et al., p. 1041.
Because Mars is so cold, almost all water on Mars is ice. What little liquid water is seeping out of Mar’s soil today is only because the water contains dissolved salts which lower the water’s freezing temperature. But those salts could only have dissolved in the water when it was a liquid. Where and when was that water liquid? On Earth before the flood.
41. Eric Hand, “Mars Rover Finds Long-Chain Organic Compounds,” Science, Vol. 347, 27 March 2015, p. 1402.
42. “... there is no reasonable astronomical scenario in which mineral grains can condense [in space].” Fred Hoyle and Chandra Wickramasinghe, “Where Microbes Boldly Went,” New Scientist, Vol. 91, 13 August 1981, p. 413.
u “Although very little is known about how the [dust] grains are formed, observations of interstellar matter indicate that the process must be very efficient; otherwise, how could the striking depletion of the refractory elements [such as silicon and magnesium] in the interstellar gas be explained?” Hubert Reeves, “Comets, Solar Wind and the D/H Ratio,” Nature, Vol. 248, 29 March 1974, p. 398.
My Translation: No one knows how dust could form in space, but dust formation must be very efficient, because few of the chemical elements needed to form dust are there. (We know that dust formed in space, because dust is in space.![]() )
)
My Response: Maybe the dust in comets came not from almost-empty space, but from Earth.
43. “As in the interstellar medium, much of the dust from comets consists of silicate minerals, but despite the similarities, there are puzzling differences. For example, interstellar dust shows the absorption signature of amorphous particles with a silicate composition, whereas Hale-Bopp and other comets have crystalline silicate, probably in the form of magnesium-rich olivine.” Dale P. Cruikshank, “Stardust Memories,” Science, Vol. 275, 28 March 1997, p. 1896. [See also pp. 1904–1909.]
u Humberto Campins and Eileen V. Ryan, “The Identification of Crystalline Olivine in Cometary Silicates,” The Astrophysical Journal, Vol. 341, 15 June 1989, pp. 1059–1066.
u “In particular, the resonance peak seen at 11.2 mm [in the impact debris from comet Tempel 1] is indicative of Mg-rich crystalline olivine.” K. J. Meech et al., “Deep Impact: Observations from a Worldwide Earth-Based Campaign,” Science, Vol. 310, 14 October 2005, p. 267.
44. Could interstellar dust, which has no crystalline pattern, have melted (or almost melted), cooled, crystallized, and then acted as condensation sites for water-ice that formed comets? Probably not. Had nonspherical dust particles melted, or almost melted, they would have become spherical due to their surface tension. Interstellar dust particles polarize starlight, so they must be elongated. Therefore, cometary dust is probably not derived from heated interstellar dust.
45. M. F. A’Hearn et al., pp. 258–264.
46. “The existence of hydrated silicates [silicates altered by liquid water] in comets is provocative, because it would suggest the presence of abundant amounts of reactive water in the formation region of the comet or in the cometary parent body.” Carey M. Lisse et al., “Spitzer Spectral Observations of the Deep Impact Ejecta,” Science, Vol. 313, 4 August 2006, p. 637.
“The presence of carbonates is provocative because, like the phyllosilicates, liquid water was thought to be required to form carbonates from CO2 in the presence of silicates.” Ibid.
u These results are “provocative” only if you didn’t realize that the material in comets came from the Earth—the water planet.
47. “ [Comet Tempel 1 is] the size of a mountain held together with the strength of the meringue in a lemon meringue pie.” Carey M. Lisse, as quoted by Ron Cowen, “Deep Impact,” Science News, Vol. 168, 10 September 2005, p. 169.
“ [The comet’s] structure is more fragile than that of a soufflť ....” Jay Melosh, as quoted by Ron Cowen, Ibid., p. 168.
48. Daniel Clery, “Rosetta Ends 2-Year Comet Mission with Final Descent,” Science, Vol. 353, 30 September 2016, p. 1482.
49. “The observation of the CO2 ice-rich spot was unexpected at these heliocentric distances, given the high volatility of CO2” G. Filacchione, et al. “Seasonal Exposure of Carbon Dioxide Ice on the Nucleus of Comet 67P/Churyumov-Gerasimenko,” Science, Vol. 354, 23 December 2016, p. 1565.
50. “In dust samples from comet Wild 2 brought back by the Stardust mission, the simplest amino acid, glycine, has been found.” Kathrin Altwegg et al., “Prebiotic Chemicals—Amino Acid [glycine] and Phosphorus—in the Coma of Comet 67P/Churyumov-Gerasimenko,” Science Advances, 27 May 2016, doi: 10.1126/sciadv.1600285, p. 2.
51. “The most abundant minerals [in comets] are the crystalline silicate minerals, olivine and pyroxene, along with troilite (FeS). These are very stable phases, common in planetary materials; however, finding them here is somewhat surprising because many expected that cometary material would be similar to interstellar material, in which most silicates are believed to be amorphous. In contrast, cometary amorphous material in the returned samples is rare or nonexistent.” Don S. Burnett, “NASA Returns Rocks from a Comet,” Science, Vol. 314, 15 December 2006, p. 1710.
52. “Current thinking suggests that it is impossible to form liquid water inside of a comet.” Dante Lauretta, as quoted by Daniel Stolte, “Frozen Comet Had a Watery Past, UA Scientists Find,” UA News, 7 April 2011.
53. Berger, Eve et al., “Evidence for Aqueous Activity on Comet 81P/Wild2 from Sulfide Mineral Assemblages in Stardust Samples and CI Chondrites,” Geochimica et Cosmochimica Acta, Vol. 75, 2011, pp. 3501–3513.
54. Fred Goesmann et al., “Organic Compounds on Comet 67P/Churyumov-Gerasimenko Revealed by COSAC Mass Spectrometry,” Science, Vol. 349, 31 July 2015, p. 497.
u Altwegg et al., pp. 1–13.
55. “Oxygen molecules are also rare in the cosmos” Christopher Crockett, “Oxygen in Comet Surprises Scientists,” Science News, Vol. 188, 28 November 2015, p. 6.
56. A. Bieler et al., “Abundant Molecular Oxygen in the Coma of 67P/Churyumov-Gerasimenko,” Nature, Vol. 526, 29 October 2015, p. 678.
57. Crockett, p. 6.
58. http://news.yahoo.com/discovery-oxygen-comet-big-surprise-181858811.html
59. Hoyle and Wickramasinghe, Lifecloud, pp. 87–113.
For two decades, these authors led a growing belief among scientists that comets are bringing cellulose, bacteria, and other organic matter to Earth.
u Hoyle and Wickramasinghe, “Where Microbes Boldly Went,” pp. 412–415.
60. Hoyle and Wickramasinghe, Lifecloud, p. 91.
61. “The cellulose strand is a complex structure, and one can wonder how a giant molecule of such a highly organized form could be present in interstellar space.” Ibid., p. 94.
62. Roland Meier et al., “A Determination of the HDO/H2O Ratio in Comet C/1995 O1 (Hale-Bopp),” Science, Vol. 279, 6 February 1998, pp. 842–844. [Similar and consistent measurements, with twice the deuterium concentration as today’s oceans, have also been made in comets Halley, Hyakutake, 2002T7, and Tuttle. However, Comet Hartley 2 has a concentration similar to Earth’s oceans. Hartley 2’s material may have been launched early in the flood (before deuterium built up in the subterranean chambers) or it may contain considerable surface water from the preflood Earth.]
u The deuterium to hydrogen ratio in Comet 67P was 3.4 times that of Earth’s water. See Kathrin Altwegg et al., “67P/Churyumov-Gerasimenko, a Jupiter Family Comet with a High D/H Ratio,” Science Online, 10 December 2014, p. 1.
u Roland Meier and Tobias C. Owen, “Cometary Deuterium,” Space Science Review, Vol. 90, Nos. 1–2, 1999, pp. 33–43.
63. A. Vidal-Madjar, “Interstellar Helium and Deuterium,” Diffuse Matter in Galaxies, editors J. Audouze et al. (Boston: D. Reidel Publishing Co., 1983), pp. 57–94.
64. Of the hundred or so important publications on this topic, the following is most recommended: Louis A. Frank with Patrick Huyghe, The Big Splash (New York: Carol Publishing Group, 1990). [See also related endnotes on page 104.]
65. “We found that there were ten times as many small comets in early November as there were in mid-January.” Frank and Huyghe, p. 187.
66. These arguments are effectively rebutted by Louis A. Frank and J. B. Sigwarth in “Atmospheric Holes: Instrumental and Geophysical Effects,” Journal of Geophysical Research, Vol. 104, No. A1, 1 January 1999, pp. 115–141.
67. “For unclear reasons, deep moonquakes seem largely confined to the side of the moon facing Earth.” Elizabeth Svoboda, “New Computers Uncover Old Quakes on the Moon,” Discover, Vol. 27, January 2006, p. 38.
u Seismometers left on the Moon during each Apollo landing recorded 12,500 seismic events. Then, in 1977, NASA turned the seismometers off. The moonquakes have now been reanalyzed using more powerful methods. Conclusion: Even after making the most adverse assumptions, most deep moonquakes were on the near side of the Moon and were clustered near the central portion of the near side. [See Yosio Nakamura, “Farside Deep Moonquakes and Deep Interior of the Moon,” Journal of Geophysical Research, Vol. 110, 18 January 2005, E01001.]
68. “Astronomers were stunned by the first images of the moon’s farside, captured by the Soviet spacecraft Luna 3 in 1959. The two hemispheres seemed like different worlds. The face we see [on Earth] has fewer large craters and far greater areas of smooth, dark, frozen lava. Nobody really knows why.” Bob Berman, “Worlds Out of Balance,” Discover, Vol. 24, December 2003, p. 38.
u “The farside, which we cannot see from Earth but has been imaged by satellites, almost completely lacks the large basaltic planes (mare) that are so prominent on the nearside.” Maria Cruz, “The Two Faces of the Moon,” Science, Vol. 338, 23 November 2012, pp. 1010–1011.
u Shadows in Figure 143 accentuate craters near the day-night boundary and minimize the appearance of craters on the near side. However, lava flows (which primarily occurred on the near side) made it smoother than the far side.
69. Mark A. Wieczarek et al., “The Crust of the Moon as Seen by GRAIL,” Science, Vol. 339, 8 February 2013, pp. 671–674.
70. A uniform ball of mass M and radius R has a moment of inertia about any diameter of 0.4000 MR2. The Moon’s polar moment of inertia is (0.3935 ± 0.0011) MR2—almost the same. [See J. O. Dickey et al., “Lunar Laser Ranging: A Continuing Legacy of the Apollo Program,” Science, Vol. 265, 22 July 1994, p. 487.] Of course, pressure and density must increase with depth. This accounts for the Moon’s moment of inertia being slightly less than that of a uniform ball. Little room is left over for a light crust. Five mascons, explained in Figure 147 on page 322, account for the major discontinuities in density within the moon.
71. “Application of gravity gradiometry to observations by the Gravity Recovery and Interior Laboratory (GRAIL) mission results in the identification of a population of linear gravity anomalies with lengths of hundreds of kilometers. Inversion of the gravity anomalies indicates elongated positive-density anomalies that are interpreted to be ancient vertical tabular intrusions or dikes formed by magmatism in combination with extension of the lithosphere ... and an increase in the Moon’s radius by 0.6 to 4.9 kilometers early in lunar history.” Jeffrey C. Andrews-Hanna et al., “Ancient Igneous Intrusions and Early Expansion of the Moon Revealed by GRAIL Gravity Gradiometry,” Science, Vol. 339, 8 February 2013, p. 675.
u Had these impacts happened slowly over thousands or millions of years (instead of within a few years), the interior heat that produced this early expansion would have dissipated.
72. Renee C. Weber et al., “Seismic Detection of the Lunar Core,” Science, Vol. 331, 21 January 2011, pp. 309–312.
73. Nicholas M. Short, Planetary Geology (Englewood Cliffs, New Jersey: Prentice-Hall, 1975), p. 87.
74. “In contrast, the far side [of the Moon] almost completely lacks maria.” Paul D. Spudis, “The New Moon,” Scientific American, Vol. 289, December 2003, p. 89.
75. “A major surprise in the early days of lunar exploration was the discovery that the soft maria visible from earth were far more rare on the moon’s farside, presumably because of some one-sided influence of the earth. Now refinements of Mariner 9 data show one hemisphere of Mars to be far rougher than the other, and Mariner 10 suggests the same asymmetry for Mercury. Data files grow, observes Bruce Murray of the California Institute of Technology, yet so does the mystery of hemispherical asymmetry. ‘We now know,’ he says, ‘a little less about the moon.’ ” Jonathan Eberhart, “The Mystery of the Hemispheres,” Science News, Vol. 105, 13 April 1974, p. 241.
76. Tiny beads of lunar basalt contain about 745 parts per million of water. As impacting comets and asteroids buried themselves deeply in what is now the Moon’s near side, the water-ice in those impactors mixed with the instantly created magma. Minutes or hours later, some of that magma erupted as a spray of droplets. Water molecules (and carbon, sulfur, chlorine, and fluorine) were diffusing out of the droplets as they solidified. [See Alberto E. Saal et al., “Volatile Content of Lunar Volcanic Glasses and the Presence of Water in the Moon’s Interior,” Nature, Vol. 454, 10 July 2008, pp. 192–194.]
u “Several studies have found concentrations of water much higher than expected in lunar materials.” Tim Elliott, “Galvanized Lunacy,” Nature, Vol. 490, 18 October 2012, p. 346.
u The D/H ratio found in apatite grains brought back by the Apollo programs matches that of comets, not Earth. [See J. P. Greenwood et al., “Water in Apollo Rock Samples and the D/H of Lunar Apatite,” Proceedings of the 41st Lunar and Planetary Science Conference, 2 March 2010, No. 2439.]
u “Concentrations of hydrogen, chlorine and sulphur in the mineral apatite from 14053 [a lunar basalt rock brought back from the moon by the Apollo 14 astronauts] are indistinguishable from apatites in common terrestrial igneous rocks.” Jeremy W. Boyce et al., “Earth-Like Lunar Apatite,” Nature, Vol. 466, 22 July 2010, p. 411.
77. M. Ozima et al., “Terrestrial Nitrogen and Noble Gases in Lunar Soils,” Nature, Vol. 436, 4 August 2005, pp. 655–659.
78. “Oxygen isotopic compositions have been found to be identical between terrestrial and lunar samples, which is inconsistent with [the belief that the moon formed by an impact of a Mars-sized body with Earth].” Junjun Zhang et al., “The Proto-Earth as a Significant Source of Lunar Material,” Nature Geoscience, Vol. 5, 25 March 2012, p. 251.
u “... the 50Ti/ 47Ti ratio of the Moon is identical to that of the Earth within about four parts per million, ... .” Ibid.
79. [Dust grains on Comet 67P] are generally rich in sodium, which explains the high sodium abundance in cometary meteoroids.” Rita Schulz et al., “Comet 67P/Churyumov-Gerasimenko Sheds Dust Coat Accumulated Over the Past Four Years,” Nature, Vol. 518, 12 February, p. 216.
80. William R. Corliss, Mysterious Universe: A Handbook of Astronomical Anomalies (Glen Arm, Maryland: The Sourcebook Project, 1979), pp. 219–239.
81. “In view of the connection of comets, meteors, and meteorites, the absence of meteorites in old deposits in the crust of the Earth is very significant. It has been estimated that at least 500 meteorites should have been found in already worked coal seams, whereas none have been identified in strata older than the Quaternary epoch (about 1-million years ago). This suggests a very recent origin [of meteors] and, by inference, of comets.” N. T. Bobrovnikoff, “Comets,” Astrophysics, editor J. A. Hynek (New York: McGraw-Hill Book Co., 1951), p. 352.
82. See “Highly Compressed Solids” on page 349.
83. Thomas C. Van Flandern, “A Former Asteroid as the Origin of Comets,” Icarus, Vol. 36, October 1978, pp. 51–74.
u Tom C. Van Flandern, Dark Matter, Missing Planets and New Comets (Berkeley, California: North Atlantic Books, 1993), pp. 185–190.
u Van Flandern built on earlier proposals by Olbers (1796) and Ovenden (1972) that a planetary breakup produced the asteroids. Van Flandern has altered his earlier paper in several ways. For example, the exploded planet was initially 90 Earth masses. Since then, his number of exploded planets has increased and their total mass has decreased.
84. Bode’s law—a mathematical curiosity, not a true law—was formulated by Johann Daniel Titius in 1766 but popularized by Johann Bode in 1772. Thus, it is often called the Bode-Titius law or the Titius-Bode law.
Bode’s law is a simple formula which gives the approximate distance of most planets from the Sun. While Bode’s law has no theoretical justification, it correctly predicted the existence and approximate orbital radius of Uranus (1781), but not Neptune (1846) and Pluto (1930). Also predicted is a planet 2.8 AU from the Sun, which closely corresponds to the average position of most asteroids. This led to the early, belief that asteroids are the remains of an exploded planet that once orbited 2.8 AU from the Sun. [For reasons given on page 338, most experts now reject this.]
Bode’s formula is
Consider how many thousands of other equally simple formulas with arbitrary numbers (corresponding to 0.4, 0.3, 2, and the values for n ) could be constructed. It should not be surprising that one of these formulas could approximate 7 of the 9 planet-Sun distances.
85. In 1668, Johannes Hevelius wrote that comets formed in the atmospheres of the giant outer planets and were flung into space by the planets’ rotation. In 1814, French mathematician Joseph Louis Lagrange proposed a more modern version of this theory. Since then, others have refined the theory, especially S. K. Vsekhsvyatsky.
u S. K. Vsekhsvyatsky, “New Evidence for the Eruptive Origin of Comets and Meteoritic Matter,” Soviet Astronomy, Vol. 2, No. 3, November–December 1967, pp. 473–484.
u S. K. Vsekhsvyatsky, “The Origin and Evolution of the Comets and Other Small Bodies in the Solar System,” The Motion, Evolution of Orbits, and Origin of Comets, editors G. A. Chebotarev and E. I. Kazimirchak-Polonskaya (New York: Springer-Verlag, 1972), pp. 413–418.
86. J. H. Oort, “The Structure of the Cloud of Comets Surrounding the Solar System, and a Hypothesis Concerning Its Origin,” Bulletin of the Astronomical Institutes of the Netherlands, Vol. 11, No. 408, 13 January 1950, pp. 91–110.
87. Oort initially estimated that 1011 comets formed 50,000 –150,000 AU away. Later, others realized that at the more distant end of that range the Sun’s gravity is so weak that passing stars, galactic clouds, and the galaxy itself would have stripped too many comets from the Oort cloud long ago. [See, for example, Julio A. FernŠndez, “Dynamical Aspects of the Origin of Comets,” The Astronomical Journal, Vol. 87, September 1982, pp. 1318–1332.] To solve this problem, more comets (1012 comets) are usually assumed to be in the cloud initially, and the cloud is assumed to be concentrated nearer the 50,000 AU end of that distance range. Others have proposed that at least 1015 comets must initially populate the Oort cloud. Oort cloud theories have many variations; only the best known are described here.
88. Jack G. Hills, “Comet Showers and the Steady-State Infall of Comets from the Oort Cloud,” The Astronomical Journal, Vol. 86, November 1981, pp. 1730 –1740.
89. Hannes Alfven and Gustaf Arrhenius, pp. 231–238.
90. For example, billiard balls are very elastic (springlike), so collisions disperse the balls. However, if the balls were made of tar (inelastic), the balls would deform or even stick together on impact, so their paths would tend to merge.
91. R. A. Lyttleton, The Comets and Their Origin (Cambridge, England: At the University Press, 1953), pp. 62–110.
92. “Although ice has been detected [in interstellar space] by its 3.1 m m absorption band, it is not nearly as abundant as expected.” P. G. Martin, McGraw-Hill Encyclopedia of Science & Technology, 6th edition (New York: McGraw-Hill Book Co., 1987), Vol. 9, p. 326.
93. “The [Mars] lander found evidence that the chemical makeup of the dust on the surface of Mars resembles that of seawater, adding to the evidence that liquid water that once may have supported life flowed on the planet’s surface.” Maggie Fox, “Mars Dust Resembles Seawater, NASA Extends Mission,” Reuters News Service, 29 September 2008,
www.news.yahoo.com/s/nm/20080929/sc_nm/us_mars_phoenix_1.
u “Phoenix’s instruments have also identified calcium carbonates in the soil. Carbonates are rocks that, on Earth, form mainly from calcium carbonates that precipitate out of seawater.” Ashley Yeager, “Racing Against the Martian Winter,” Science News, www.sciencenews.org/view/generic/id/36595/title/ Racing_against_the_Martian_ winter, 10 October 2008.
u “Among the greatest surprises was the discovery ... [on Mars] of calcium carbonate (at concentrations of 5 percent) ... . [Calcium carbonate] is a very common mineral on Earth. ... Others have since spotted isolated outcrops of calcium carbonate rocks although other types of carbonates seem more common.” Peter H. Smith, “Digging Mars,” Scientific American, Vol. 305, November 2011, p. 53.
"The Origin of Limestone" chapter on pages 259–264 explains why most limestone originated on Earth. Most of it precipitated (out-salted) from the supercritical subterranean water and was swept upward during the flood. Indeed, the pH of Mars’ limestone was “nearly the same as ocean water on Earth.” Ibid.
u “... we have identified a compositional unit on Mars that contains a mineralogical component likely attributable to chloride salts. We initially identified these deposits because of their spectral distinctiveness ... The deposits range in area from ~1 km 2 to ~25 km 2 [at about 200 locations] and generally are topographically lower than the immediate surrounding terrain.” M. M. Osterloo et al., “Chloride-Bearing Materials in the Southern Highlands of Mars,” Science, Vol. 319, 21 March 2008, p. 1651.
u “ [The Mars Rover named Opportunity, operating in what appears to be a dried-up water channel,] has uncovered soil that is more than half salt, adding to the evidence for Mars’ wet past.” Guy Webster, “Mars Rovers Break Driving Records, Examine Salty Soil,” Jet Propulsion Laboratory News Release, 2 March 2005, p. 1.
u “Some rocks [on Mars] may be as much as 40 percent salt, he notes. ‘That’s an astonishing amount’ and could result only from a briny solution soaking through rock and then evaporating, leaving the salt behind, Clark says.” Benton Clark, as quoted by Ron Cowen, “Red Planet Makes a Splash,” Science News, Vol. 165, 6 March 2004, p. 147.
u “... the identification of halite at the martian surface indicates extreme salinity ...” Nicholas J. Tosca et al., “Water Activity and the Challenge for Life on Early Mars,” Science, Vol. 320, 30 May 2008, p. 1205.
u “The presence of hydrated minerals on the surface of Mars implies that the crust was once altered by the action of liquid water. ... the degree of alteration of the ancient martian crust is more extensive than previously assumed.” J. Carter et al., “Detection of Hydrated Silicates in Crustal Outcrops in the Northern Plains of Mars,” Science, Vol. 328, 25 June 2010, pp. 1610. See also pp. 1682–1686.
94. Colin M. Dundas, et al., “Exposed Subsurface ice Sheets in the Martian Mid-Latitudes,” Science, Vol. 359, 12 January 2018, pp. 199–201.
95. Many claim that comets had to begin outside the orbit of Mars where typically (a) temperatures are cold enough for frost to condense on dust particles in space, and (b) the Sun’s ultraviolet radiation is unlikely to break water molecules apart. This belief overlooks two considerations.
First, if water vapor condensed as frost on dust particles beyond Mars, then frost should be commonly detected on asteroids in the asteroid belt. Frost is seldom observed. Second, icy particles orbiting beyond Mars, will not, in general, form a comet. Long periods of time increase the chances of water vapor and ice particles disintegrating.
However, the fountains of the great deep would quickly form comets. Water molecules would not have to be brought together; they would start together. Dirt, ice, gases, and other unlikely chemicals in comets would not need to be found and mixed uniformly together; they also would start together.
96. “Our next worry arose because the condensation of water-ice grains in interstellar clouds of low density presented severe conceptual problems. For ice crystals less than a micrometre in size to form in a pure gas, ‘condensation nuclei’, about which the crystals grow, must form at an adequate rate. ... Another early objection we had against the ice-grain theory was that calculations based on this model could not reproduce the way in which the fogging, or extinction, of starlight varied with wavelength: ... Secondly, attempts to find the strong absorption band at 3.1 m m due to water ice in the spectra of heavily obscured stars consistently failed.” Hoyle and Wickramasinghe, “Where Microbes Boldly Went,” p. 412.
97. Zdenek Sekanina, “Detection of a Satellite Orbiting the Nucleus of Comet Hale Bopp (C/1995 O1),” First International Conference on Comet Hale Bopp, Puerto de la Cruz, Tenerife, Canary Islands, Spain, 2–5 February 1998.
98. H. D. P. Lee, Aristotle: Meteorologica (Cambridge, Massachusetts: Harvard University Press, 1952), p. 43.
99. Thomas H. Corcoran, Seneca: Natural Quaestiones (Cambridge, Massachusetts: Harvard University Press, 1972), pp. 227–299.
100. Previously, faulty logic (traceable to the time of Aristotle) went as follows: Because bodies (stars) beyond the Moon do not change their appearance, and a comet changes weekly, comets must not lie beyond the Moon.
101. M. E. Bailey et al., “The Origin of Comets,” Vistas in Astronomy, Vol. 29, 1986, p. 61.
102. Peter Lancaster-Brown, Halley’s Comet & the Principia (Aldeburgh, England: Aries Press, 1986), p. 17.
103. On 1 March 1665, Samuel Pepys entered in his famous diary the following statement:
At noon I [went] to dinner at Trinity House, and thence to Gresham College, where Mr. Hooke read a second very curious lecture about the late Comet; among other things proving very probably that this is the very same Comet, that appeared before in the year 1618, and that in such a time probably it will appear again, which is a very new opinion; but all will be in print. Samuel Pepys, The Diary of Samuel Pepys, editor Henry B. Wheatley, Vol. 4, Part 2 (New York: Croscup & Sterling Co., 1946), p. 341.
Pepys later became the president of The Royal Society (of London), the prestigious scientific body that hosted the above lecture. The idea that some comets reappear was “a very new opinion” and deserves credit for originality. While no periodic comets were visible between 1609 and 1677, Robert Hooke may have suggested the possibility to later researchers, such as Edmond Halley. Halley’s correct prediction in 1705 of the return of the comet of 1682 (later called Halley’s comet) in 1758 was one of science’s classic achievements. However, Halley was criticized for making a prediction that would not be tested until after his death, “when he could no longer be embarrassed.”
104. Newton, “That the Comets Are Higher Than the Moon, and in the Regions of the Planets,” Proposition XXXIX, Lemma IV, Book III, Principia, pp. 399–401.
105. Fred L. Whipple, “Discovering the Nature of Comets,” Mercury, Vol. 15, January–February 1986, p. 5.
106. Richard A. Proctor, “Comet Families of the Giant Planets,” Knowledge: A Monthly Record of Science, Vol. 6, 4 July 1884, p. 5.
u Richard A. Proctor, “The Capture Theory of Comets,” Knowledge: A Monthly Record of Science, Vol. 6, 8 August 1884, pp. 111–112, 126–128.
107. “Thus, cometary nuclei could not have condensed in situ at distances exceeding 100 AU ... Direct condensation of the comets in situ, at the great distances of their aphelia in Oort’s sphere, is not possible.” Ernst J. ÷pik, “Comets and the Formation of Planets,” Astrophysics and Space Science, Vol. 21, 1973, pp. 320, 394.
108. Thomas M. Donahue, “Comment on the Paper ‘On the Influx of Small Comets into the Earth’s Upper Atmosphere II. Interpretation’ by L. A. Frank et al.,” Geophysical Research Letters, Vol. 13, June 1986, pp. 555–557.
109. “There are at least 70,000 Trans-Neptunian Objects (TNOs) with diameters larger than 100 km in the 30-50 AU region.” Lorenzo Iorio, “Dynamical Determination of the Mass of the Kuiper Belt from Motions of the Inner Planets of the Solar System,” Monthly Notices of the Royal Astronomical Society, Vol. 375, 2007, p. 1311.
110. This high improbability can be shown two ways. First, the “back-of-the-envelope” method. The Marsden-Williams Comet Catalogue (Cambridge, Massachusetts: Minor Planet Center, 1996, pp. 10–41) lists 774 different sightings of non-periodic comets. One can select two out of 774 different objects 299,151 ways, or ![]() . Five numbers (i, q, e, w, and W) specify an ellipse in space. Let’s say that the chance that two randomly-selected comet sightings have “similar” values for the combination q and e is 0.25—at least as similar as those of the “strange pairs.” Two angles (W and w ) have values ranging from 0 to 360 degrees and a third angle, i, ranges between 0 and 180 degrees. If each comet sighting in a “strange pair” had values for i, W , and w within five degrees on either side of the corresponding angles of the other comet, one might expect about three “strange pairs” simply due to chance—nine less than the twelve actually observed.
. Five numbers (i, q, e, w, and W) specify an ellipse in space. Let’s say that the chance that two randomly-selected comet sightings have “similar” values for the combination q and e is 0.25—at least as similar as those of the “strange pairs.” Two angles (W and w ) have values ranging from 0 to 360 degrees and a third angle, i, ranges between 0 and 180 degrees. If each comet sighting in a “strange pair” had values for i, W , and w within five degrees on either side of the corresponding angles of the other comet, one might expect about three “strange pairs” simply due to chance—nine less than the twelve actually observed.
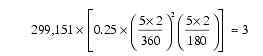
111. My computer simulations of the solar system during its last 350 years have shown that Herschel-Rigollet did not come near enough to any planet for that gravity boost. Therefore, its gravity boost probably came from mass beyond 30 AU.
A more accurate approach involves a computer simulation. By examining the 30 recorded consecutive orbits of Halley’s comet, one can see that planetary perturbations change certain orbital elements less than others. (For example, i—the angle of inclination—changes very little from orbit to orbit.) Therefore, changes in each orbital element must be weighted properly when comparing two different orbits.
Next, for all 774 comet sightings, I swapped each true orbital element with the corresponding orbital element of a randomly chosen comet. Then, a count was made of how many of the 299,151 random pairings were as similar as the “strange pairs.” Typically, there were three. In other words, chance can explain about three of the twelve “strange pairs” shown on page 318. That leaves about ten other pairs—or ten comets that were seen on two consecutive orbits.
This is surprising, because the estimated periods for both members of each pair are too large for them to be the same comet. However, these comets spend most of their time far beyond the planets. Some very slight force, accelerating the comets for centuries, could greatly shorten their periods.
112. The Great Comet of 1680 was also blamed for the Biblical deluge. In 1694, Edmond Halley suggested that the global flood may have been due “to an Earth-comet encounter.”
Two years later, Isaac Newton’s successor in the Lucasian professorship at Cambridge, William Whiston (1667–1752), published a book, A New History of the Earth, stating that “on Friday, November 28, 2349 B.C., the Great Comet of 1680 passed very close to the Earth. The near collision caused a tidal breakup of the Earth’s crust. The subsequent release of subterranean waters, together with precipitation from the comet’s atmosphere and tail, caused the Biblical deluge by raising a tide several miles high.” [See Yeomans, p. 164.]
William Whiston is also famous for his popular translation (1732) from Greek of The Complete Works of Flavius Josephus. Josephus was the Jewish-Roman historian and Jewish military leader of Galilee who witnessed the Roman distruction of Jerusalem in A.D. 70. Josephus’ voluminous writings have given us detailed secular insights into the lives of the Jewish people in the centuries before the sacking of Jerusalem.
113. K. J. Meech, and O. R. Hainaut, “HST Imaging of Distant Comet Nuclei,” Bulletin of the American Astronomical Society, Vol. 29, July 1997, p. 1021.
114. P. M. Muller and W. L. Sjogren, “Mascons: Lunar Mass Concentrations,” Science, Vol. 161, 16 August 1968, pp. 680–684.
115. Richard A. F. Grieve, “The Record of Impact on Earth,” Geological Society of America, Special Paper 190, 1982, pp. 25–37.
116. The energy required just to “disperse” a planet of uniform density, mass M, and radius R can be shown to be
![]()
where G is the gravitational constant. If the planet’s density is greater in its core, as it is for all planets, the energy requirement increases. “Disperse” here means to accelerate each of the planet’s particles to its escape velocity.
117. Bill Napier and R. J. Dodd, “The Missing Planet,” Nature, Vol. 242, 23 March 1973, pp. 250 – 251.
A planet could explode if it contained enough fissionable material that suddenly became a critical mass. However, as Anders notes, “such an explosion 6 million years ago [or less] would have left large amounts of long-lived radioactivity, such as 10Be and 53Mn, on the Earth, Moon, and meteorites.” These isotopes have not been detected. [See E. Anders, Discussions of “A Former Major Planet of the Solar System,” Comets, Asteroids, Meteorites, editor A. H. Delsemme (Toledo, Ohio: The University of Toledo, 1977), p. 479.]
118. Jupiter generates tidal friction inside Io, which produces Io’s heat. [See Ron Cowen, “Close Encounter: Galileo Eyes Io,” Science News, Vol. 156, 11 December 1999, pp. 382–383.]
119. S. K. Vsekhsvyatsky, “Comets and the Cosmogony of the Solar System,” Comets, Asteroids, Meteorites, editor A. H. Delsemme (Toledo, Ohio: The University of Toledo, 1977), p. 470.
120. Ariel A. Roth, “Some Questions about Geochronology,” Origins, Vol. 13, No. 2, 1986, p. 75.
121. Marsden and Sekanina, “On the Distribution of ‘Original’ Orbits of Comets of Large Perihelion Distance,” The Astronomical Journal, Vol. 78, December 1973,p. 1123.
122. FernŠndez, pp. 1318, 1324.
123. Paul R. Weissman, “The Oort Cloud and the Galaxy: Dynamical Interactions,” The Galaxy and the Solar System, editors Roman Smoluchowski et al. (Tucson, Arizona: The University of Arizona Press, 1986), p. 212.
124. Some researchers have suspected that one of two stars, Algol or Gliese 710, may have recently disturbed an Oort cloud. Actual measurements dispute this. “The new figures reveal that neither star comes close enough to shake up the Oort Cloud and generate a comet shower.” Ron Cowen, “Dino Death: A Stellar Weapon,” Science News, Vol. 153, 31 January 1998, p. 79.
u Jeffrey Winters, “A Brief Tour of a Bad Cosmic Neighborhood,” Discover, Vol. 19, April 1998, p. 56.
125. “Any Oort cloud formed with the Sun and planets 4.5 billion years ago would by now have been devastated ...” Victor Clube and Bill Napier, “Close Encounters with a Million Comets,” New Scientist, 15 July 1982, p. 149.
126. Julio A. FernŠndez, “The Formation of the Oort Cloud and the Primitive Galactic Environment,” Icarus, Vol. 129, September 1997, pp. 106–119.
u Everhart, p. 329.
127. FernŠndez, “Dynamical Aspects of the Origin of Comets,” p. 1318.
128. Any giant planet would expend much of its orbital energy in flinging 10,000 Earth masses of comets out toward an Oort cloud. Also, the gravity-assisted boosts needed to give so many comets their angular momentum would shrink the planet’s orbit, requiring it to have begun much farther from the Sun.
While this might help solve one aspect of the comet origin problem, it creates problems for the few astronomers trying to figure out how the giant planets evolved. These astronomers wonder how the giant planets could form where they are now, even if billions of years were available. That problem worsens for objects trying to form farther from the Sun, where matter is more spread out and moving even more slowly. [See ÷pik, pp. 307–398. Also see Richard Greenberg, “The Origin of Comets Among the Accreting Outer Planets,” Dynamics of Comets: Their Origin and Evolution, editors Andrea Carusi and Giovanni B. Valsecchi (Boston: D. Reidel Publishing Co., 1985), pp. 3–10.]
129. S. Alan Stern and Paul R. Weissman, “Rapid Collisional Evolution of Comets during the Formation of the Oort Cloud,” Nature, Vol. 409, 1 February 2001, pp. 589–591.
130. “No wastage would occur with Uranus or Neptune, but then the ejection time scale, 1011 yr, is prohibitive. ” ÷pik, p. 395.
131. Gerard P. Kuiper, “On the Origin of the Solar System,” Astrophysics, editor J. A. Hynek (New York: McGraw-Hill Book Co., 1951), pp. 357–424.
132. Ron Cowen, “Second Look Finds No Comet Reservoir,” Science News, Vol. 149, 22 June 1996, p. 395.
133. Weissman, p. 210.
134. John F. Kerridge and James F. Vedder, “An Experimental Approach to Circumsolar Accretion,” Symposium on the Origin of the Solar System (Paris, France: Centre National de la Recherche Scientifique, 1972), pp. 282–283.
135. Martin Harwit, Astrophysical Concepts (New York: John Wiley & Sons, 1973), pp. 394–395.
136. Thomas S. Kuhn, The Structure of Scientific Revolutions (Chicago: The University of Chicago Press, 1970). [Both the National Review and the Modern Library (a division of Random House) listed this book among the hundred best nonfiction books written in English during the 20th century.]